Abstract
Objective
To develop and standardize the Limb and Oral Apraxia Test (LOAT) for Korean patients and investigate its reliability, validity, and clinical usefulness for patients with stroke.
Methods
We developed the LOAT according to a cognitive neuropsychological model of limb and oral praxis. The test included meaningless, intransitive, transitive, and oral praxis composed of 72 items (56 items on limb praxis and 16 items on oral praxis; maximum score 216). We standardized the LOAT in a nationwide sample of 324 healthy adults. Intra-rater and inter-rater reliability and concurrent validity tests were performed in patients with stroke. We prospectively applied the LOAT in 80 patients and analyzed the incidence of apraxia. We also compared the clinical characteristics between the apraxia and non-apraxia groups.
Results
The internal consistency was high (Cronbach’s alpha=0.952). The inter-rater and intra-rater reliability and concurrent validity were also high (r=0.924–0.992, 0.961–0.999, and 0.830, respectively; p<0.001). The mean total, limb, and oral scores were not significantly different according to age and education (p>0.05). Among the 80 patients with stroke, 19 (23.8%) had limb apraxia and 21 (26.3%) had oral apraxia. Left hemispheric lesions and aphasia were significantly more frequently observed in the limb/oral apraxia group than in the non-apraxia group (p<0.001).
Apraxia is described as a disorder affecting the execution of learned movement that cannot be explained by a motor or sensory deficit, incomprehension, or inattention to commands [1,2]. Apraxia is a common neurologic deficit after brain damage, and the prevalence of apraxia has been reported to be variable. It is estimated that up to 70% of patients with left hemispheric stroke and 30% of patients with right hemispheric stroke experience apraxia during the subacute period [3]. Apraxia causes severe deterioration of the rehabilitation process after stroke and, consequently, severe decline in the ability to perform activities of daily living [4]. Therefore, it is important to evaluate praxis abilities in patients with stroke. However, apraxia is difficult to understand and study because of confusing terminology, remaining doubts on this disease, and the absence of a standardized apraxia test [5-7].
In the praxis model proposed by Gonzalez-Rothi et al. [2] in 1991, motor praxis can be generated using verbal and visual stimuli (object or gesture). Upon the application of these stimuli, the information is analyzed phonologically and visually, and sequentially enters into the phonological and action input lexicon. Thereafter, the action semantic system interprets the information meaningfully, and final motor responses are selected and revised through the action output lexicon and gestural buffer. However, some information can be generated without cognitive processes, through what is referred to as ‘the visuomotor conversion mechanism’.
The many different forms of apraxia can be classified according to type (e.g., ideomotor or ideational apraxia), task (e.g., dressing apraxia), lesion (e.g., callosal apraxia), effector (e.g., limb or oral apraxia), or modality (e.g., optical or tactile apraxia). Among the various types of apraxia, ideomotor apraxia is the classic model [3]. Ideomotor apraxia refers to impairment in executing the use of a tool or gesture, such that patients with ideomotor apraxia experience difficulty in performing appropriate actions related to verbal commands, visually presented tools, or imitating another person’s gestures. Ideational apraxia is believed to be linked to damage in the dominant posterior parietal cortex that involves semantic memory impairment. There are two additional types of apraxia associated with the orofacial muscles: oral motor apraxia and apraxia of speech. Apraxia of speech is a motor speech disorder affecting the translation of conscious speech plans into motor plans, resulting in limited and difficult formation of sounds and words [8]. Unlike apraxia of speech, oral motor apraxia means impairment of non-speech vocal tract gestures [9].
Several assessments have been developed for the diagnosis of apraxia and the evaluation of patients with this disorder; however, few studies have used standardized comprehensive apraxia tests [10]. Many apraxia tests were developed in Western countries. Therefore, these tests may not be suitable for application in other countries because of language and cultural differences [11-15]. A comprehensive apraxia test for stroke and other brain disorders in Korean patients is not yet available.
The primary aim of this study was to develop a new comprehensive test for limb and oral apraxia (Limb and Oral Apraxia Test [LOAT]) that has good reliability and validity based on a cognitive neuropsychological model of limb praxis [2] and that is suitable for Korean patients with brain disorders. We also investigated the usefulness of the test in Korean patients with stroke.
The LOAT was developed in accordance with well-accepted guidelines for test development and cultural adaptation [11,12,16]. First, we organized a committee for the development of the LOAT. The committee members included 4 physiatrists, 1 neuropsychologist, and 1 speech-language pathologist. We adopted the cognitive neuropsychological model of praxis developed by Gonzalez-Rothi et al. [2] to develop a new test for limb and oral apraxia. According to this model, final motor responses can be generated using verbal and visual stimuli (objects or gestures). Therefore, we chose three types of stimulus items for limb and oral apraxia: verbal, gesture, and visual object.
We thoroughly reviewed previously published screening and comprehensive apraxia tests, including the Test of Oral and Limb Apraxia [17], Frenchay Aphasia Screening Test-Revised [18], Apraxia Battery for Adults-2 [19], apraxia screen of the Test of Upper Limb Apraxia [20], and apraxia subtest of the Western Aphasia Battery (WAB) [21]. All pooled items were given a score of 0 ‘inappropriate’, 1 ‘partially appropriate’, or 2 ‘appropriate’ by each committee member, and only the 82 items that were scored 2 points by all the committee members were selected as items of the preliminary version of the LOAT. The committee also added new items considering specific cultural and language differences in Korea. Among the new items, only those with a content validity index of >0.7 were selected, and 30 items were added to the preliminary version of the LOAT.
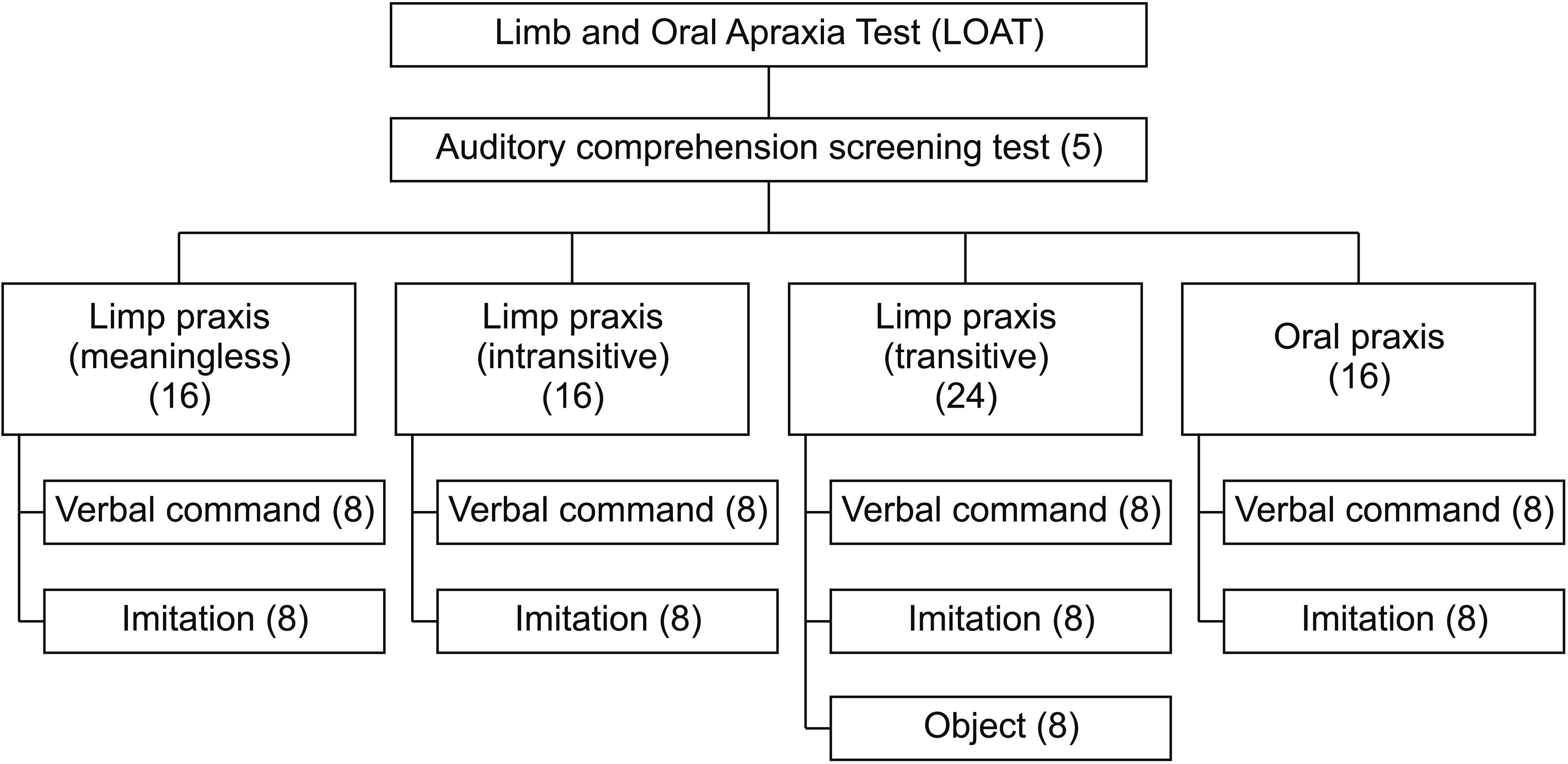
Finally, a total of 112 items were pooled for an interitem consistency reliability test, and these items were distributed throughout the subdomains of the LOAT (meaningless, intransitive, and transitive gesture). We designed a 4-point scoring system that enables qualitative analysis: 3 ‘normal’, 2 ‘adequate’ (target motor responses possible but with hesitance, delay, groping, and self-correction), 1 ‘partially adequate’ (within the target motor response but with use of the hand as a tool or with an incorrect direction), and 0 ‘inadequate’ (no response, out of the target response, or perseveration). The final LOAT items were selected from the 112 preliminary items based on internal consistency assessed using Cronbach’s alpha. A total of 72 items (56 items for limb apraxia, 16 items for oral apraxia) were selected for the standardization of the LOAT. Therefore, the maximum scores for limb and oral apraxia are 168 and 48 points, respectively. The final test domains and number of LOAT items are shown in Fig. 1, and the details of the test items are provided in Appendix 1.
The results of apraxia tests can be misleading owing to cognitive impairment and aphasia. Therefore, we also developed a 5-item auditory comprehension screening test as a pretest for the LOAT. The pretest items are hierarchically arranged from simple to complex sentences, and the response options are ‘yes’ (1 point) or ‘no’ (0 point).
We recruited healthy adults for inter-item consistency testing and standardization of the LOAT. The inclusion criteria were as follows: (1) age ≥20 years; (2) >1 standard deviation of the score in the age-matched Korean version of the Mini-Mental State Examination (K-MMSE); and (3) no history of brain injury, psychiatric illness, or other neurological illness that can affect apraxia tests. A total of 324 participants were recruited nationwide. We collected demographic data, Edinburgh Handedness Inventory scores, and K-MMSE scores. The LOAT was administered to the participants by physiatrists, occupational therapists, speech-language pathologists, and psychologists who had completed education and training for the administration and scoring of the LOAT. The test results were analyzed according to age groups (decades) and education groups.
To verify the reliability and validity of the LOAT, 11 patients with brain disorders who met the following inclusion criteria were enrolled: (1) a diagnosis of subacute supratentorial stroke (within 2 months of onset) based on brain imaging (computed tomography or magnetic resonance imaging), (2) ability to understand the instructions of the apraxia test (≥3 correct responses out of 5 in the auditory comprehension screening pretest), and (3) no prior cognitive impairment or psychiatric illness. Patients with infratentorial lesions or subarachnoid hemorrhage were excluded.
For the intra-rater and inter-rater reliability test, we recorded movie clips of the responses of the 11 patients while applying the LOAT. Nine medical professionals, including medical doctors and speech-language pathologists, scored each patient’s performance after watching the video. A correlation analysis was performed for interrater reliability. For test-retest reliability, we provided the same video clips to the same medical professionals 2 weeks later and performed a correlation analysis between the initial and second LOAT scores. To test concurrent validity, as there are no published standardized apraxia tests in Korean, we selected the subtest score of apraxia from the Korean version of the WAB (K-WAB). A correlation analysis was performed between the LOAT and K-WAB subtest scores in 17 patients with brain disorders.
We prospectively administered the LOAT and collected data on patients with stroke from the Department of Physical Medicine and Rehabilitation of Anam Hospital from May 2012 to February 2015. The inclusion and exclusion criteria for these patients were identical to those mentioned above. Demographic data (age, sex, education, and handedness) and neurological data (time after onset, lesion side, National Institutes of Health Stroke Scale [NIHSS] score, K-MMSE score, and presence of aphasia) were collected. The presence of aphasia was determined using the Korean version of the Frenchay Aphasia Screening Test or the K-WAB. During the administration of the LOAT, the patients were instructed to gesture with the non-dominant hand unless that hand was not hemiplegic. Patients with a score of <2 standard deviations from the standardized reference value of the LOAT were classified into the apraxia group (cutoff score of limb apraxia=154.01, oral apraxia=42.0). We obtained informed consent from the participants. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (No. 2014AN0330).
We performed statistical analysis using SPSS Statistics version 20 software (IBM, Armonk, NY, USA). A p-value of <0.05 was considered significant. Internal consistency was measured using Cronbach’s alpha. A correlation analysis between LOAT scores and age or education was performed using Pearson correlation, and intra-rater and inter-rater reliability and validity tests were conducted using Spearman correlation. Post-hoc analysis of LOAT scores was performed according to age and education groups of healthy participants. The Mann-Whitney U-test and the chi-square test were used to analyze differences between the apraxia and non-apraxia groups.
A total of 324 healthy participants (113 males, 211 females) were enrolled for the standardization of the LOAT. The mean age of the participants was 51.0 years, and the mean education duration was 11.7 years. The mean K-MMSE score was 28.0 points. The place of residence with the highest number of participants was Gyeongsang Province, followed by Chungcheong Province and Seoul. The general characteristics of the participants are shown in Table 1.
The mean total score of the LOAT was 212.4 (out of 216). The mean scores of the limb and apraxia subscales were 165.2 (out of 168) and 47.2 (out of 48), respectively. Pearson correlation showed no significant correlation between age, education, and LOAT total and subscale scores, except for the transitive semantic process (age: r=-0.151, p= 0.006; education: r=0.136, p=0.014). Further, post-hoc analysis of LOAT scores showed no significant differences between the age and education groups (Table 2). The percentile values and relevant LOAT scores derived from 324 healthy participants are shown in Appendix 2.
The internal consistency of the LOAT was high, and the Cronbach’s alpha value for the total items was 0.952. The Cronbach’s alpha value was 0.942 for limb apraxia and 0.821 for oral apraxia. The inter-rater reliability was also very high (r=0.949–0.989 for total, r=0.946–0.992 for limb apraxia, r= 0.924–0.970 for oral apraxia; p<0.001). The intra-rater reliability was 0.982–0.999, 0.964–0.999, and 0.961–0.999 for the total score, limb apraxia, and oral apraxia, respectively (p<0.001). In the assessment of concurrent validity, the correlation coefficients between the LOAT and K-WAB scores were 0.830 for the total scores (p<0.001), 0.840 for limb apraxia (p<0.001), and 0.699 for oral apraxia (p=0.002).
We initially selected 168 patients. A total of 80 patients (mean age, 63.09 years) were eventually enrolled based on the inclusion criteria. Among the 80 patients, 47 were men and 61 had ischemic stroke. The clinical characteristics are shown in Table 3.
Of the 80 patients, 19 (23.8%) had scores <2 standard deviations in limb apraxia and 21 (26.3%) had oral apraxia. Fifty-five patients (68.8%) had neither limb apraxia nor oral apraxia (Table 4). Among the variables, the hemispheric site of stroke, presence of aphasia, NIHSS score, and K-MMSE score were significantly different between the limb apraxia and non-apraxia groups. Among the 19 patients with limb apraxia, 16 (84.2%) had left hemispheric or bi-hemispheric stroke and 15 (78.9%) had aphasia. On the other hand, among the 61 patients with no limb apraxia, 31 (50.8%) had left hemispheric stroke and 12 (19.7%) had aphasia. Furthermore, cognitive function, which was screened using the K-MMSE, and stroke severity, which was measured with the NIHSS, was worse in the limb apraxia group (p<0.001). No significant differences were found in age, sex, time after onset (in days), handedness, or education level between the groups (Table 5). Similar results were found in the oral apraxia group (Table 4).
We investigated the correlation of apraxia severity with the limb or oral apraxia score. The K-MMSE and NIHSS scores showed a strong correlation with the apraxia score in the apraxia groups (limb apraxia group: K-MMSE r=0.747, p<0.001 and NIHSS r=-0.733, p<0.001; oral apraxia group: K-MMSE r=0.678, p=0.001 and NIHSS r=-0.674, p=0.001). However, in the non-apraxia group, the K-MMSE and NIHSS scores did not correlate with the apraxia score. The apraxia test score (limb or oral score) and the K-WAB score showed statistically significant correlations in the limb or oral apraxia group and in the non-oral apraxia group in patients with left brain damage (limb apraxia group: r=0.845, p<0.001; no limb apraxia group: r=0.355, p=0.177; oral apraxia group: r=0.704, p=0.001; no oral apraxia group r=0.695, p=0.004).
We aimed to develop a new comprehensive apraxia test battery for patients with stroke that would be suitable for the Korean culture and language. The LOAT was successfully developed in accordance with well-accepted guidelines and standardized in 324 healthy adults from a nationwide sample. The reliability and validity tests for the LOAT showed high internal consistency and very high inter-tester and intra-tester reliability. The concurrent validity for patients with stroke was also high. After the administration of the LOAT to 80 patients with stroke, the results indicated that the LOAT is a reliable and valid assessment tool for apraxia in patients with stroke.
The neural basis of apraxia remains controversial. According to previous studies on apraxia [2,22], the praxic pathway consists of auditory and visuomotor conversion mechanisms. These mechanisms involve the conversion of external visual information into internal neural commands appropriate for the desired motion. Motor planning is crucial in this conversion. Inadequate conversion results in performance errors that might increase the complexity of the movement. After the initial recognition, meaningful gestures are processed along the semantic route, whereas meaningless gestures are processed along the structural route only.
It has been reported that apraxia occurs in 30%–70% of patients with left brain damage and in 8%–30% of patients with right brain damage [3]. The results of the present study showed that 31.2% of patients with stroke had either limb or oral apraxia. Considering the time after the onset of stroke, this incidence is similar to that in previous reports. Further, left lateralized lesions and the presence of aphasia were highly associated with limb and oral apraxia in patients with stroke. Many studies have suggested that, in humans, praxic function shares the same functional neuroanatomy with language function [23-25], and the severity of aphasia has a strong correlation with purposeful movement [26]. Previous studies have revealed that both language and praxis tasks activate the inferior frontal gyrus and supramarginal gyrus. Broca’s region seemed to be related to semantic processinglinked language and meaningful action. However, premotor, precentral, and postcentral gyrus lesions seemed to be more related to praxic function than to language function [25]. One previous study reported that most patients with ideomotor apraxia also had aphasia, and, conversely, many aphasic patients had apraxia [23].
In the present study, the K-MMSE and NIHSS scores showed strong correlations with the apraxia test scores in the apraxia groups. Therefore, we can assume that apraxia is associated with the severity of stroke. Furthermore, according to logistic regression analysis, the most important contributing factor for apraxia was the K-MMSE score. This result is compatible with that of a previous study that revealed the relation between apraxia and cognitive function [27].
Although apraxia is a common cognitive-motor deficit after stroke, its assessment and treatment have yet to be fully addressed in the clinical setting. Furthermore, there are no reliable and valid assessment tools in the Korean language. The present study found that the LOAT has several strengths and advantages. First, the LOAT is a newly developed, comprehensive apraxia battery for Korean patients with brain disorders. The LOAT was developed in accordance with well-accepted guidelines and adapted for Korean culture [11,12]. Moreover, standardization was completed in a relatively large population of healthy adults from a nationwide sample, and our results confirmed that the LOAT is a reliable and valid test for patients with stroke. Second, the LOAT consists of test items with high content validity and internal consistency. Moreover, these items were evenly distributed according to the stimuli (verbal, gesture, and object) and semantic process (meaningless, transitive, and intransitive) based on well-known conceptual models of apraxia. Furthermore, the LOAT is based on a 0–3 scoring system, which makes it possible to evaluate the quality aspects of motor responses such as groping, hesitation, and using the hand as a tool. Third, the LOAT can be easily applied clinically in both inpatient and outpatient settings for patients with stroke or brain disorders. The LOAT requires 15–20 minutes to administer. Any medical professional, including medical doctors, occupational or physical therapists, speech-language pathologists, and psychologists, can easily apply the LOAT after undergoing training on the administration and scoring of tests and measures. Either the limb or oral test can be applied for evaluation purposes. Fourth, the individual LOAT scores are not dependent on age or education. Most cognitive and language tests are strongly influenced by age and education; therefore, the interpretation of test results need careful consideration of these two factors. However, our correlation and post-hoc analysis results showed that in a healthy population, there were no significant differences between age groups or education groups. Therefore, the individual test scores of the limb, oral, or total LOAT can simply be compared with the provided reference values or percentile values. Lastly, the LOAT has an additional 5-item auditory comprehension screening test as a pretest. Similar to many other cognitive tests, the evaluation of apraxia can be affected by language function, especially auditory comprehension. If a patient has severe auditory comprehension deficit, the test results can be misleading. Our results suggest that a score of ≥3 (out 5 points) may indicate eligibility for a full test administration. Therefore, this screening pretest may help in the optimal selection of examinees and in the interpretation of test results.
A limitation of this study is that there is no gold standard for the diagnosis of apraxia, which hinders the assessment of the sensitivity and specificity of the LOAT. Therefore, instead of absolute cutoff values for diagnosing apraxia, we provided reference tables of mean and standard deviation and percentile values. These may help facilitate the clinical and research use of the LOAT in patients with apraxia.
Apraxia is a common cognitive-motor deficit after stroke. The LOAT is a newly developed comprehensive limb and oral apraxia test for Korean patients with brain disorders, especially for patients with stroke. The LOAT has high internal consistency, reliability, and validity and can be easily administered to patients after stroke. This test may aid in the diagnosis and rehabilitation of patients with apraxia and can be employed in future stroke research.
ACKNOWLEDGMENTS
This work was supported by the R&D grant (No. 2012301) on rehabilitation from the Korea National Rehabilitation Center Research Institute, Ministry of Health & Welfare, and the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science and ICT) (No. 2019R1A2C2003020).
REFERENCES
1. Rothi LJ, Heilman KM, Watson RT. Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry. 1985; 48:207–10.

2. Gonzalez Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cogn Neuropsychol. 1991; 8:443–58.

3. Foundas AL, Henchey R, Gilmore RL, Fennell EB, Heilman KM. Apraxia during Wada testing. Neurology. 1995; 45:1379–83.

4. Foundas AL, Macauley BL, Raymer AM, Maher LM, Heilman KM, Gonzalez Rothi LJ. Ecological implications of limb apraxia: evidence from mealtime behavior. J Int Neuropsychol Soc. 1995; 1:62–6.

5. De Renzi E, Faglioni P, Sorgato P. Modality-specific and supramodal mechanisms of apraxia. Brain. 1982; 105(Pt 2):301–12.

6. Goldenberg G, Hermsdorfer J, Spatt J. Ideomotor apraxia and cerebral dominance for motor control. Brain Res Cogn Brain Res. 1996; 3:95–100.

7. Koski L, Iacoboni M, Mazziotta JC. Deconstructing apraxia: understanding disorders of intentional movement after stroke. Curr Opin Neurol. 2002; 15:71–7.

9. Chang SE, Kenney MK, Loucks TM, Poletto CJ, Ludlow CL. Common neural substrates support speech and non-speech vocal tract gestures. Neuroimage. 2009; 47:314–25.

10. Dovern A, Fink GR, Weiss PH. Diagnosis and treatment of upper limb apraxia. J Neurol. 2012; 259:1269–83.

11. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000; 25:3186–91.

12. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993; 46:1417–32.

13. Lee JS, Suh KT, Kim JI, Lee HS, Goh TS. Validation of the korean version of the neck pain and disability scale. Asian Spine J. 2013; 7:178–83.

14. Ha JW, Pyun SB, Lee HY, Hwang YM, Nam K. Reliability and validity analyses of the Korean version of Frenchay Aphasia Screening Test in brain-damaged patients. Korean J Commun Disord. 2009; 14:46–57.
15. Kim SJ, Shin JC, Kim DY, Kim H. Korean version of Stroke and Aphasia Quality of Life Scale-39 (KSAQOL-39): its validity and reliability. J Rehabil Res. 2012; 16:245–65.
16. Kim DY, Pyun SB, Kim EJ, Ryu BJ, Choi TW, Pulvermuller F. Reliability and validity of the Korean version of the Communicative Activity Log (CAL). Aphasiology. 2016; 30:96–105.

17. Helm-Estabrooks N. TOLA: Test of Oral and Limb Apraxia. Chicago, IL: Riverside Publishing Company;1992.
18. Enderby P, Crow E. Frenchay aphasia screening test: validity and comparability. Disabil Rehabil. 1996; 18:238–40.

19. Dabul B. Apraxia battery for adults. Austin, TX: Pro-ED;2000.
20. Vanbellingen T, Kersten B, Van de Winckel A, Bellion M, Baronti F, Muri R, et al. A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). J Neurol Neurosurg Psychiatry. 2011; 82:389–92.

21. Kertesz A. Western aphasia battery test manual. New York, NY: Grune & Stratton;1982.
22. Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010; 48:3855–67.

23. Papagno C, Della Sala S, Basso A. Ideomotor apraxia without aphasia and aphasia without apraxia: the anatomical support for a double dissociation. J Neurol Neurosurg Psychiatry. 1993; 56:286–9.

24. Vry MS, Tritschler LC, Hamzei F, Rijntjes M, Kaller CP, Hoeren M, et al. The ventral fiber pathway for pantomime of object use. Neuroimage. 2015; 106:252–63.

25. Weiss PH, Ubben SD, Kaesberg S, Kalbe E, Kessler J, Liebig T, et al. Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct Funct. 2016; 221:563–76.

Table 1.
General characteristics of healthy subjects (n=324)
Table 2.
Limb and oral apraxia test scores according to age and education groups (n=324)
Table 3.
General characteristic of stroke patients (n=80)
Table 4.
Comparison of variables according to presence or absence of oral apraxia (n=80)
| Variable | Oral apraxia (n=21) | No apraxia (n=59) | p-value |
|---|---|---|---|
| Age (yr) | 61.76±14.05 | 63.56±13.45 | 0.55 |
| Gender | 0.228 | ||
| Male | 10 | 37 | |
| Female | 11 | 22 | |
| Education (yr) | 10.19±4.71 | 9.53±5.07 | 0.91 |
| Handedness | 0.91 | ||
| Right | 20 | 56 | |
| Left | 0 | 2 | |
| Bilateral | 1 | 1 | |
| K-MMSE (30) | 16.05±8.14 | 25.90±3.72 | <0.001* |
| NIHSS | 8.71±4.24 | 4.73±3.64 | <0.001* |
| Location of stroke | 0.001* | ||
| Right | 3 | 29 | |
| Left | 14 | 30 | |
| Bilateral | 4 | 0 | |
| Aphasia | <0.001* | ||
| Present | 17 | 10 | |
| Absent | 4 | 49 | |
| Time between onset and LOAT (day) | 25.52±20.05 | 20.41±17.26 | 0.27 |
| LOAT score | |||
| Limb apraxia (168) | 125.05±40.81 | 163.29±6.59 | <0.001* |
| Oral apraxia (48) | 33.00±10.68 | 47.07±1.35 | <0.001* |
| Total score (216) | 158.67±50.53 | 210.42±7.29 | <0.001* |
Table 5.
Comparison of variables according to presence or absence of limb apraxia
| Variable | Limb apraxia (n=19) | No apraxia (n=61) | p-value |
|---|---|---|---|
| Age (yr) | 65.11±12.93 | 62.46±13.78 | 0.490 |
| Gender | 0.248 | ||
| Male | 9 | 38 | |
| Female | 10 | 23 | |
| Education (yr) | 9.95±4.98 | 9.63±4.99 | 0.827 |
| Handedness | 0.057 | ||
| Right | 18 | 58 | |
| Left | 0 | 2 | |
| Bilateral | 1 | 1 | |
| K-MMSE (30) | 14.84±7.77 | 25.95±3.54 | <0.001* |
| NIHSS | 9.32±4.11 | 4.67±3.56 | <0.001* |
| Location of stroke | <0.001* | ||
| Right | 3 | 29 | |
| Left | 12 | 32 | |
| Bilateral | 4 | 0 | |
| Aphasia | <0.001* | ||
| Present | 15 | 12 | |
| Absent | 4 | 49 | |
| Time between onset and LOAT (day) | 25.00±18.57 | 20.74±17.92 | 0.319 |
| LOAT score | |||
| Limb apraxia (168) | 117.63±38.04 | 164.34±3.71 | <0.001* |
| Oral apraxia (48) | 33.47±11.98 | 46.46±2.67 | <0.001* |
| Total (216) | 152.00±49.33 | 210.80±5.39 | <0.001* |
Appendices
Appendix 1.
Limb and Oral Apraxia Test (LOAT
1) Limb apraxia test (56)
(1) Meaningless gesture (16)
(2) Intransitive gesture
(3) Transitive gesture




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download