2. Watchie J. Cardiovascular and pulmonary physical therapy: a clinical manual. 2nd ed. London: Elsevier Health Sciences;2010.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (update 2015). Fontana, WI: Global Initiative for Chronic Obstructive Lung Disease;2015.
4. Wouters EF. Chronic obstructive pulmonary disease. 5. Systemic effects of COPD. Thorax. 2002; 57:1067–70.
5. Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, et al. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med. 2010; 104:829–39.

7. Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003; 40(5 Suppl 2):25–33.

8. Roberts SE, Stern M, Schreuder FM, Watson T. The use of pursed lips breathing in stable chronic obstructive pulmonary disease: a systematic review of the evidence. Phys Ther Rev. 2009; 14(4):240–6.

9. Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 10:CD008250.

10. Lewis A, Cave P, Stern M, Welch L, Taylor K, Russell J, et al. Singing for lung health: a systematic review of the literature and consensus statement. NPJ Prim Care Respir Med. 2016; 26:16080.

11. Mayer AF, Karloh M, Dos Santos K, de Araujo CL, Gulart AA. Effects of acute use of pursed-lips breathing during exercise in patients with COPD: a systematic review and meta-analysis. Physiotherapy. 2018; 104:9–17.

12. Cahalin LP, Braga M, Matsuo Y, Hernandez ED. Efficacy of diaphragmatic breathing in persons with chronic obstructive pulmonary disease: a review of the literature. J Cardiopulm Rehabil. 2002; 22:7–21.

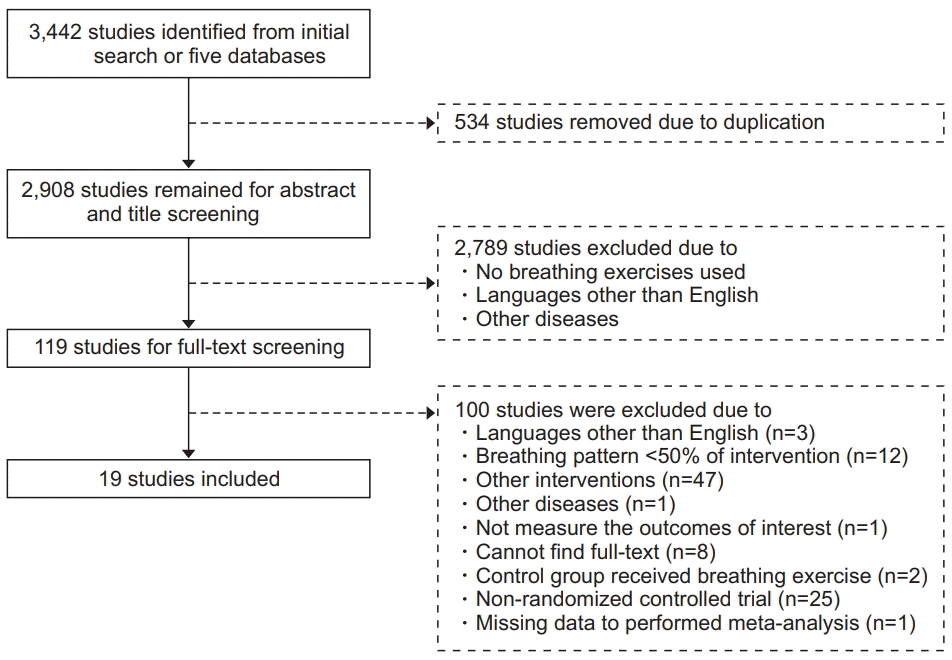
13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9, W64.

14. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) [Internet]. London: The Cochrane Collaboration;2011. [cited 2019 Jul 1]. Available from:
https://handbook-5-1.cochrane.org/.
15. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines. 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–6.

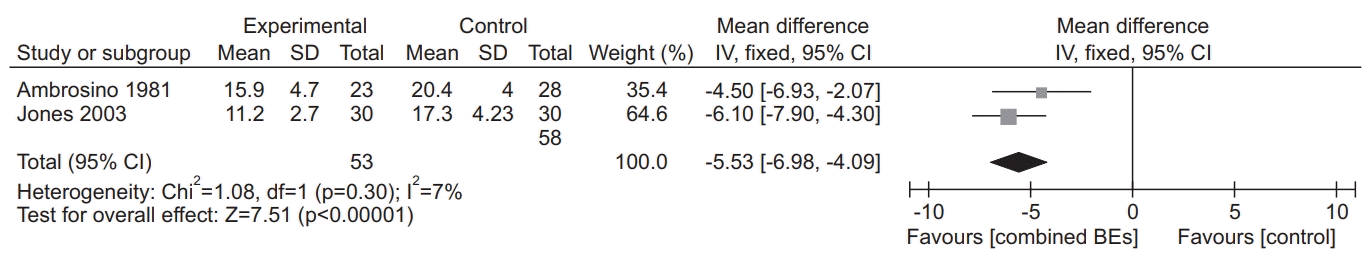
16. Ambrosino N, Paggiaro PL, Macchi M, Filieri M, Toma G, Lombardi FA, et al. A study of short-term effect of rehabilitative therapy in chronic obstructive pulmonary disease. Respiration. 1981; 41:40–4.

17. Tiep BL, Burns M, Kao D, Madison R, Herrera J. Pursed lips breathing training using ear oximetry. Chest. 1986; 90:218–21.

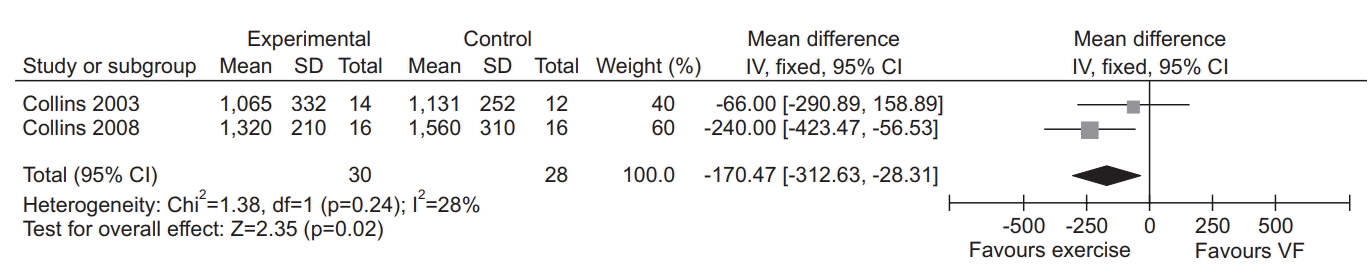
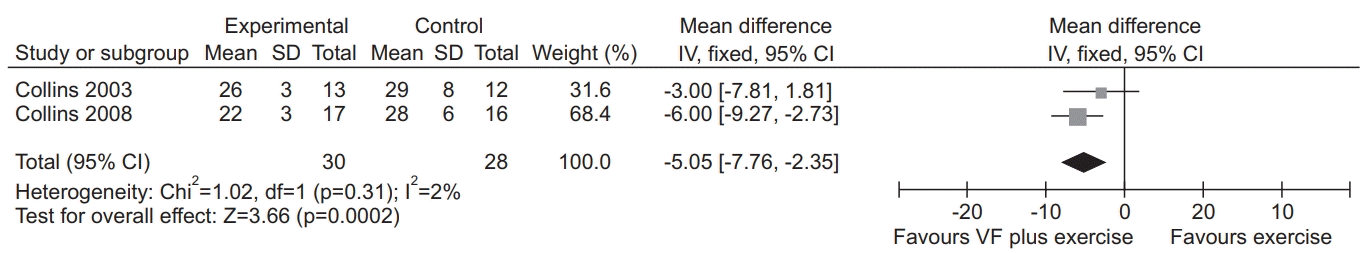
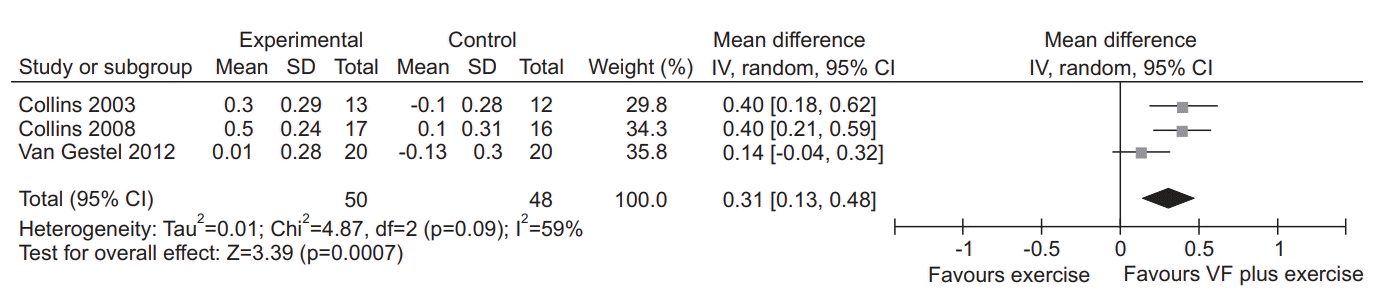
18. Collins EG, Fehr L, Bammert C, O’Connell S, Laghi F, Hanson K, et al. Effect of ventilation-feedback training on endurance and perceived breathlessness during constant work-rate leg-cycle exercise in patients with COPD. J Rehabil Res Dev. 2003; 40(5 Suppl 2):35–44.

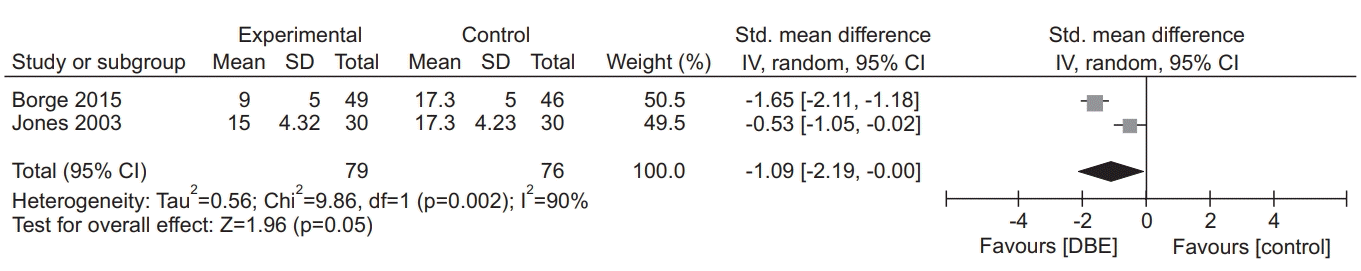
19. Jones AY, Dean E, Chow CC. Comparison of the oxygen cost of breathing exercises and spontaneous breathing in patients with stable chronic obstructive pulmonary disease. Phys Ther. 2003; 83:424–31.

20. Garrod R, Dallimore K, Cook J, Davies V, Quade K. An evaluation of the acute impact of pursed lips breathing on walking distance in nonspontaneous pursed lips breathing chronic obstructive pulmonary disease patients. Chron Respir Dis. 2005; 2:67–72.
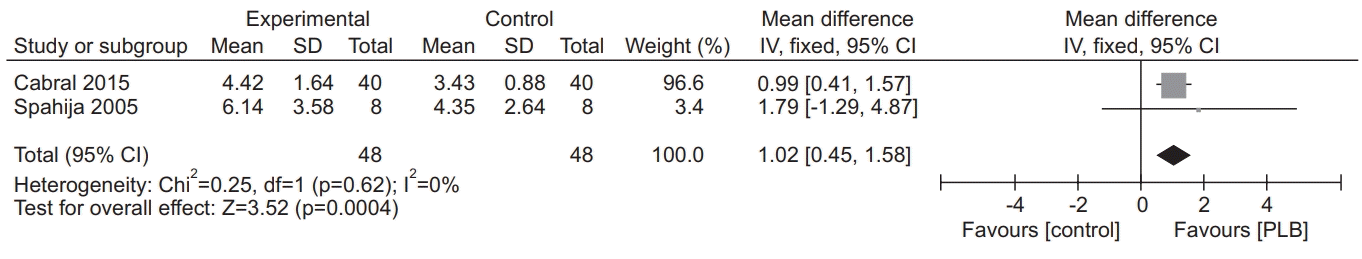
21. Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005; 128:640–50.

22. Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev. 2007; 27:237–44.
23. Collins EG, Langbein WE, Fehr L, O’Connell S, Jelinek C, Hagarty E, et al. Can ventilation-feedback training augment exercise tolerance in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2008; 177:844–52.

24. Faager G, Stâhle A, Larsen FF. Influence of spontaneous pursed lips breathing on walking endurance and oxygen saturation in patients with moderate to severe chronic obstructive pulmonary disease. Clin Rehabil. 2008; 22:675–83.

25. Bonilha AG, Onofre F, Vieira ML, Prado MY, Martinez JA. Effects of singing classes on pulmonary function and quality of life of COPD patients. Int J Chron Obstruct Pulmon Dis. 2009; 4:1–8.
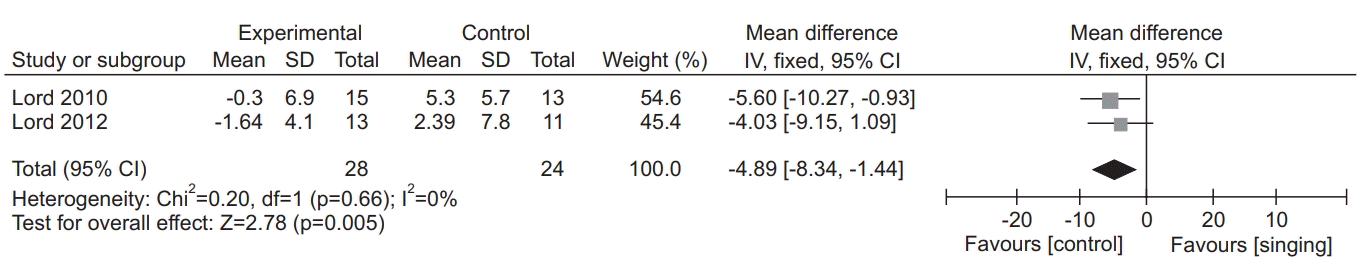
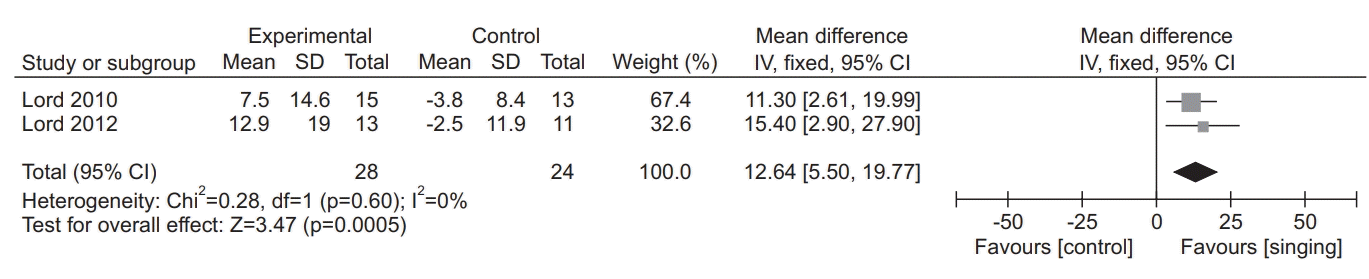
26. Lord VM, Cave P, Hume VJ, Flude EJ, Evans A, Kelly JL, et al. Singing teaching as a therapy for chronic respiratory disease: a randomized controlled trial and qualitative evaluation. BMC Pulm Med. 2010; 10:41.

27. Lin WC, Yuan SC, Chien JY, Weng SC, Chou MC, Kuo HW. The effects of respiratory training for chronic obstructive pulmonary disease patients: a randomised clinical trial. J Clin Nurs. 2012; 21:2870–8.

28. Lord VM, Hume VJ, Kelly JL, Cave P, Silver J, Waldman M, et al. Singing classes for chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulm Med. 2012; 12:69.

29. van Gestel AJ, Kohler M, Steier J, Teschler S, Russi EW, Teschler H. The effects of controlled breathing during pulmonary rehabilitation in patients with COPD. Respiration. 2012; 83:115–24.

30. Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Arch Phys Med Rehabil. 2012; 93:571–7.

31. Bhatt SP, Luqman-Arafath TK, Gupta AK, Mohan A, Stoltzfus JC, Dey T, et al. Volitional pursed lips breathing in patients with stable chronic obstructive pulmonary disease improves exercise capacity. Chron Respir Dis. 2013; 10:5–10.

32. de Araujo CL, Karloh M, Dos Reis CM, Palu M, Mayer AF. Pursed-lips breathing reduces dynamic hyperinflation induced by activities of daily living test in patients with chronic obstructive pulmonary disease: a randomized cross-over study. J Rehabil Med. 2015; 47:957–62.

33. Cabral LF, D’Elia Tda C, Marins Dde S, Zin WA, Guimaraes FS. Pursed lip breathing improves exercise tolerance in COPD: a randomized crossover study. Eur J Phys Rehabil Med. 2015; 51:79–88.
34. Borge CR, Mengshoel AM, Omenaas E, Moum T, Ekman I, Lein MP, et al. Effects of guided deep breathing on breathlessness and the breathing pattern in chronic obstructive pulmonary disease: a doubleblind randomized control study. Patient Educ Couns. 2015; 98:182–90.

35. Kant S, Singh GV. Breathing exercises as adjuvant in the management of COPD: an overview. Lung India. 2006; 23(4):165–9.
36. Gigliotti F, Coli C, Bianchi R, Romagnoli I, Lanini B, Binazzi B, er al. Exercise training improves exertional dyspnea in patients with COPD: evidence of the role of mechanical factors. Chest. 2003; 123:1794–802.
37. Rabe KF. Improving dyspnea in chronic obstructive pulmonary disease: optimal treatment strategies. Proc Am Thorac Soc. 2006; 3:270–5.

38. Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002; 31:115–23.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download