INTRODUCTION
To understand the motor control of the hand in patients with congenital mirror movements (CMMs), investigation of motor organization in distal hand muscles is important to develop viable interventions to improve patient outcomes. However, forearm and arm muscles also contribute to hand motor function and must be considered in these cases. Most studies concerning CMMs have been focused on the motor organization in the distal hand muscles only, and confirmed the dominance of the ipsilateral corticospinal pathways in those muscles. To the best of our knowledge, there is scarce data on forearm and arm muscles, and no data on the lower extremities in these cases [
1].
In a previous study, we probed the ipsilateral corticospinal pathway in a patient with CMMs by examining neurophysiologic findings for the distal hand muscles [
2]. In this study, we aimed to investigate the motor organization of the forearm and arm muscles as well as the distal hand muscles and lower extremities in the same patient, as evaluated via a transcranial magnetic stimulation (TMS) study repeated over a period of 10 years. We hoped by utilizing this approach that we would be in the position to gain an insight into the pathophysiology of CMMs and also the impact of development on the disorder.
CASE REPORT
The patient first visited our hospital at the age of 9, presenting with mirror movements [
2]. It was revealed that involuntary symmetrical movements occurred in the opposite hand when he moved his hand. Therefore, he had difficulty in tying his shoelace and fastening a button such as on a shirt. He received occupational therapy and went to a general school, but his educational achievement was very low. At the age of 19, his mirror movements were more reduced than had been noted before. He was able to perform dissociated movements with his hands such as typing and assembling bolts and nuts. However, he still displayed a marked level of clumsiness and low grip strength in both hands. In the 9-hole pegboard test, it was noted that he took 26 seconds for each hand (normal: 16.41±1.65 seconds for the right hand, 17.53±1.73 seconds for the left hand) and his grip strength for each hand was 26 kg (normal: 43.1–49.0 kg for each hand).
His mirror movements were recently reassessed according to the Woods and Teuber scale [
3]. In this case, the mirror movements were persistently observed in the upper extremities, particularly the distal hands, but not in the lower extremities. The intensity of mirror movements was decreased at age of 19, but their frequency was not reduced. The scores for lifting a finger, opening and clenching the fist, sequential finger-thumb opposition, flexing the wrist, and abducting the arm were all noted at an evaluated number 1, where they were 2 or 3 at the age of 9.
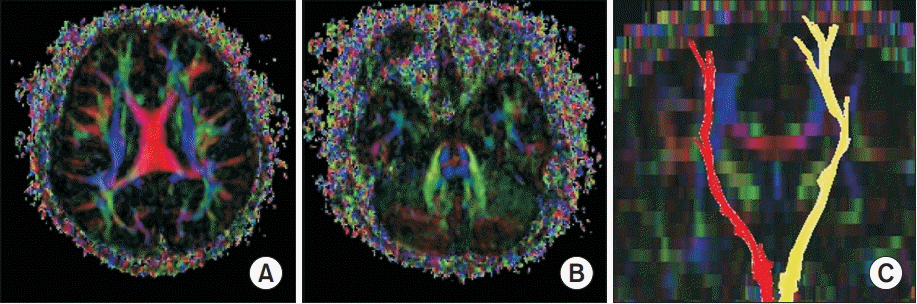
Equally important, it is noted that the brain magnetic resonance imaging showed no abnormal findings. Similarly, the diffusion tensor images (DTI) were acquired using a Verio 3.0T system (Siemens, Erlangen, Germany) equipped with a 12-channel sensitivity encoding (SENSE) head coil for single-shot echo planar imaging. The imaging parameters utilized were: echo time=93 ms, repetition time=7,900 ms, field of view=230 mm×230 mm, sampling matrix size=128×128 reconstructed with homodyne processing to 256×256, SENSE factor=3, EPI=128, and bvalue=1,000 s/mm
2. As a result, we acquired 47 contiguous, 3.0 mm thick slices parallel to the anterior commissure-posterior commissure line in 30 different diffusion directions. We therefore performed a tractography on the basis of fiber assignment by continuous tracking (FACT). The thresholds of the tracking termination were noted at 0.2 for the fractional anisotropy (FA) and 60° for the angle. Furthermore, for fiber tracking of the corticospinal tract (CST), two region of interest (ROI) were drawn on color-coded two-demensional FA map. For this purpose, a seed ROI was drawn at the CST portion in the anterior mid-pons and the target ROI in the anterior lower pons. As has been noted, the diffusion tensor tractography demonstrated normal symmetrical crossed CST (
Fig. 1).
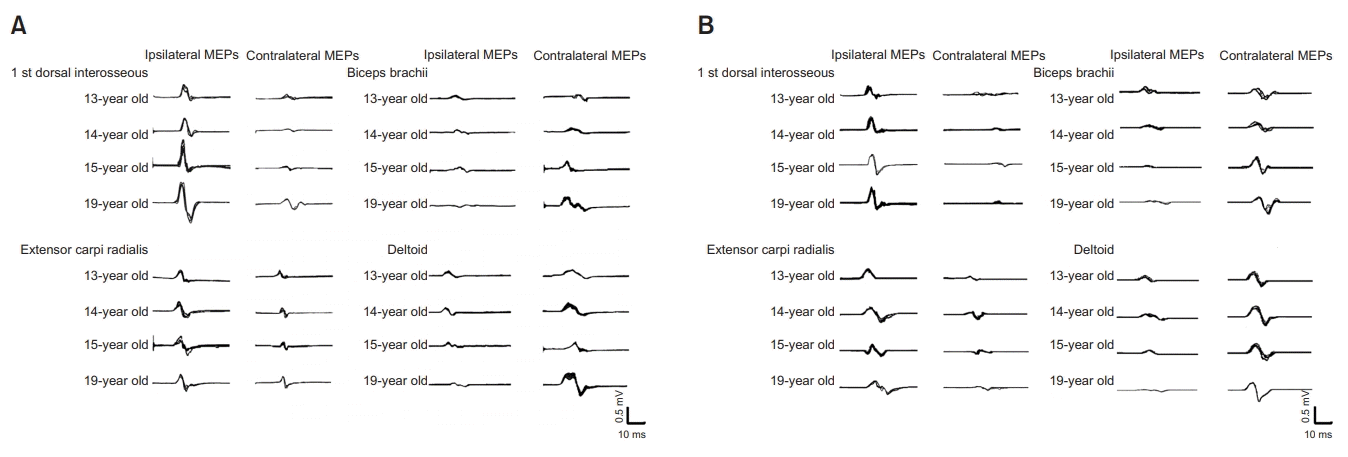
In this study, we performed a TMS study spanning 10 years from aged 9 to 19. The possible physical and psychological complications from the study were explained to the patient, who gave written informed consent to participate in the study. The TMS system utilized was a MagPro (MagVenture, Lucernemarken, Denmark), and figure-8 type magnetic coils (70 mm in diameter) were used to stimulate the primary motor cortex. The recordings of ipsilateral motor evoked potentials (iMEPs) and contralateral motor evoked potentials (cMEPs) were made simultaneously at the bilateral first dorsal interosseous (FDI), extensor carpi radialis (ECR), biceps brachii (BB), deltoid, tibialis anterior, gastrocnemius and vastus medialis. Additionally, the FDI, ECR, and BB plus deltoid represent the distal hand, forearm, and arm muscles, respectively. In this case, the stimulation intensity was set at 110% of the resting motor threshold. It is noted that each hemisphere was stimulated four times, and the shortest latency and average peak to peak amplitudes were used for analysis.
In this case, iMEPs were evoked from all upper extremity muscles. At the FDI and ECR the amplitude and latency of iMEPs were noted as being higher and shorter than those of cMEPs (
Table 1). Therefore, the ratio of the amplitude of the iMEPs to the cMEPs (iMEP/cMEP) was greater than 1 at these points (
Table 2). Additionally, the reverse case was observed in the BB and deltoid muscles. At the BB and deltoid the amplitude and the latency of iMEPs were lower and longer than those of cMEPs (
Table 1). It is concluded that there were no ipsilateral motor evoked responses in the lower extremities (
Table 3).
The iMEPs in distal hand muscles increased in amplitude as the patient grew older, despite the decrement in intensity of the evaluated mirror movements. In the arm muscles, however, the cMEPs were seen to have increased in amplitude. Likewise, for forearm muscles, iMEPs were persistently dominant even though the iMEP/cMEP ratio was lower than for the distal hand muscles (
Fig. 2).
In this study, we performed whole exome sequencing to look for gene mutations that have been associated with CMMs, specifically
DCC, RAD51, PROK2, SEMA3A, SOX10, FGF8, CEP152, CHD7, FGFR1, TACR3, KISS1R, NSMF, PROKR2, WDR11, CREBBP, EP300 , HS6ST1 , HESX1, and
GDF6 [
4]. However, it is noted that no pathogenic variant relating to CMMs was found, and there was no family history of mirror movements, which reflects that this case is considered as a sporadic situation.
DISCUSSION
To begin with, the iMEPs of distal hand muscles became more dominant as the patient grew older. In the arm muscles, however, it is noted that the cMEPs increased in amplitude. In those cases, the iMEP/cMEP ratio was smaller in the forearm than in the distal hand, but the amplitudes of iMEPs were still larger than those of cMEPs. That being said, there was no evidence of an uncrossed corticospinal projection which was observed in the lower extremities.
In a previous TMS study of healthy children, we reported that cMEPs of distal hand muscles were noted and observed in infants. However, the cMEPs were not elicited in arm muscles, even in some children over 12 years of age. Moreover, the distal hand muscles showed a relevant amplitude increase at an earlier age than that of the arm muscles. This is a result of the late maturation of the CST projection to the proximal muscles [
5]. This noted result could also be a factor in the age-related changes in the dominance of cMEPs, such as was revealed in the arm muscles that were observed in this study. Our TMS results showed that the suppression of iMEPs effects and makes cMEPs stronger, an effect that is mediated by the normal developmental pathway in the arm muscles. However, the dominance of iMEPs rather than cMEPs in the distal hand muscles represents an example of opposite changes of the motor organization pattern, as compared to the normal developmental pathway.
In our earlier report, we concluded that the iMEPs of the distal hand in our patient did not reflect the existence of a branch of a crossed CST, but rather was shown to be more characteristic of an uncrossed CST [
2]. In this study we found that although the intensity of mirror movements was reduced as the patient grew older, the iMEPs of the distal hands became stronger. This finding is compatible with the previous reviewed report [
1]. Moreover it suggests that an uncrossed CST represented by iMEPs are problematic for motor control, because they cannot be integrated with a crossed sensory system in that case [
6].
The patient in this case had no other congenital deformity, unlike a patient we previously reported on with the characteristics of axial mesodermal dysplasia syndrome [
7]. We also found that there was no genetic abnormality related to the CMMs. Although the two cases involved different causes of motor organization abnormalities, the same motor organization pattern was manifested including in this case an uncrossed corticomotoneuronal projection. This suggests that a review of the TMS studies may be more useful than only a review of genetic studies in the work confirming a diagnosis of CMM disorder.
Our TMS study challenges the validity of the
Kanga mouse model of CMM disorder, which was previously used for a genetic study [
8]. The
Kanga mice show a unique hopping gait which reflects an uncrossed CST in the lower extremities, but our patient showed only crossed CST as characterized in the lower extremities. In addition we also question a recent DTI study which reported the existence of only an uncrossed CST in a patient with CMMs [
9]. However, we confirmed not only the existence of an uncrossed CST, but also a crossed corticospinal projection to the upper extremities in our patient. Thus, further studies of more patients are definitely needed to reveal the underlying cause of CMMs.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download