INTRODUCTION
MATERIALS AND METHODS
Literature search
Study selection
Data extraction
Assessment of methodological quality
Statistical analysis
RESULTS
Eligible studies and characteristics
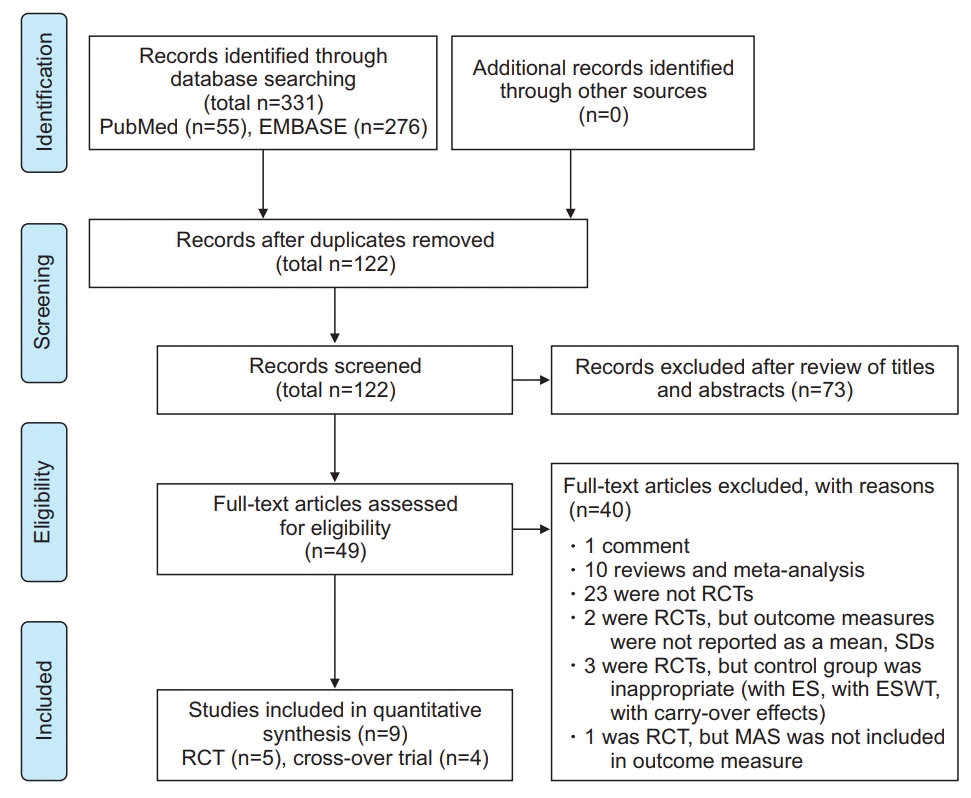 | Fig. 1.Preferred Reporting Items for Systemic Reviews and MetaAnalysis (PRISMA) flow chart. RCT, randomized controlled trial; ES, electrical stimulation; ESWT, extracorporeal shockwave therapy; MAS, Modified Ashworth Scale. |
Table 1.
| Study (year) |
Participants |
Intervention |
Comparison |
Outcome |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion | No. of patients (treated/control) |
Duration of illness (mo) |
ESWT energy (mJ/mm2) | Mode | Application focus | Treated muscle | Total counts (shots) | Tested muscle |
MAS (baseline) |
MAS |
Follow-up time points (after the end of sessions) | ||||
| Treated | Control | Treated | Control | Treated | Control | ||||||||||
| Taheri et al. [23] (2017) | Stroke | 14/14 | 33±21.4 | 25.8±9.9 | 0.1 | NA | Myotendinous junction | GCM | 4500 | GCM | 2.6±0.5 | 2.5±0.5 | 1.8±0.5 (immediately after) | 2.1±0.7 (immediately after) | Immediately after, 9 weeks after |
| Yoon et al. [24] (2017) | Stroke | 54/26 (elbow flexor) | 100.3±98.3a) | 63.5±94.1c) | 0.068–0.093 | NA | Muscle belly, myotendinous junction | BB, semitendinosus | 4500 | BB | 2.8±0.7a) | 2.6±0.6c) | 2.6±0.8a) | 2.6±0.6c) | Immediately after |
| 66.8±51.9b) | 2.9±0.5b) | 2.7±0.6b) | |||||||||||||
| 26/28 (knew flexor) | 99.1±85.1d) | 38.7±30.2f) | Semitendinosus | 2.9±1.0d) | 2.4±0.7f) | 2.4±0.8d) | 2.4±0.7f) | Immediately after | |||||||
| 51.1±36.0e) | 2.9±0.6e) | 2.3±0.6e) | |||||||||||||
| Dymarek et al. [13] (2016) | Stroke | 30/30 | 51.30±25.46 | 51.53±26.13 | 0.03 | Radial | Muscle belly | FCR, FCU | 1500 | Elbow | 1.5±0.5 | 1.6±0.7 | 1.3±0.7 (immediately after) | 1.6±0.8 (immediately after) | Immediately after, 1 hr after, 24 hr after |
| RC | 1.7±0.7 | 1.8±0.9 | 1.3±0.5 (immediately after) | 1.7±0.8 (immediately after) | |||||||||||
| FF | 2.1±0.9 | 1.7±0.7 | 1.5±0.8 (immediately after) | 1.7±0.7 (immediately after) | |||||||||||
| Marinelli et al. [16] (2015) | Multiple sclerosis | 34/34 | NA | NA | Radial | Muscle belly, myotendinous junction | GCM, soleus, achilles tendon | 8000 | Ankle extensor | 2.7±0.8 | 2.6±1.0 | 2.6±0.9 | 2.5±1.1 (4 weeks after) | 1 week after, 4 weeks after | |
| Tirbisch [25] (2015) | Stroke | 4/4 | 0.75–6 | 0.03 | NA | Myotendinous junction | GCM, soleus | 18000 | GCM | 3.0±0.8 | 1.8±0.9 | 1.4±0.5 | 1.5±1.7 | Immediately after | |
| Soleus | 2.9±1.0 | 1.5±1.2 | 1.5±1.3 | 1.1±1.4 | Immediately after | ||||||||||
Values are presented as mean±standard deviation.
ESWT, extracorporeal shockwave therapy; MAS, Modified Ashworth Scale; NA, not applicable; GCM, gastrocnemius; PT, physiotherapy; BB, biceps brachii; FCR, flexor carpi radialis; FCU, flexor carpi ulnaris; RC, radio-carpal joint; FF, finger flexor joint.
Table 2.
| Study (year) |
Participants |
Intervention |
Comparison |
Outcome |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion | No. of patients | Duration of illness | ESWT energy (mJ/mm2) | ESWT mode | Application focus | Treated muscle | Total counts (shots) | Tested muscle | MAS (baseline) | MAS | Follow-up time points | |
| Moon et al. [26] (2013) | Stroke | 30 | 80.5±46.5 days | 0.089 | NA | Myotendinous junction | GCM | 4500 | GCM | 2.5±0.7 | 1.8±0.6 (4 weeks after) | Immediately after, 1 week after, 4 weeks after |
| Gonkova et al. [14] (2013) | Cerebral palsy | 25 | 4.84±3.11 years | NA | Radial | Muscle belly | GCM, soleus | 1500 | Ankle extensor | 2.7±0.1 | 2.2±0.1 (4 weeks after) | Immediately after, 2 weeks after, 4 weeks after |
| Amelio and Manganotti [15] (2010) | Cerebral palsy | 12 | 8.0±2.31 years | 0.03 | NA | Muscle belly | GCM, soleus | 1500 | Ankle extensor | 3.3±0.5 | 2.8±0.6 (12 weeks after) | Immediately after, 1 week after, 4 weeks after, 12 weeks after |
| Manganotti and Amelio [12] (2005) | Stroke | 20 | Stroke ≥9 months | 0.03 | NA | Muscle belly, myotendinous junction | Hypertonic forearm muscles, interosseus of hands | 4700 | Wrist flex- | 3.4±0.7 | 3.0±0.5 (12 weeks after) | Immediately after, 1 week after, 4 weeks after, 12 weeks after |
| Finger flexors | 3.2±0.6 | 1.8±0.7 (12 weeks after) | ||||||||||
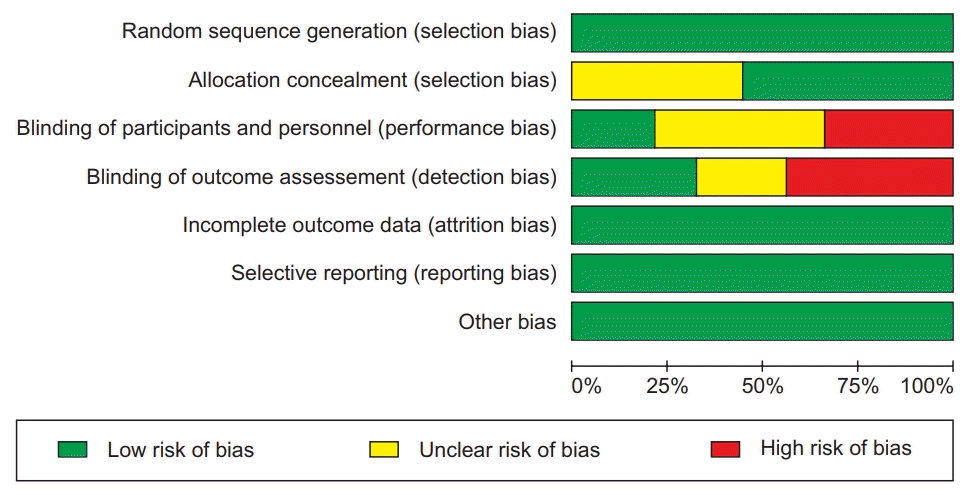
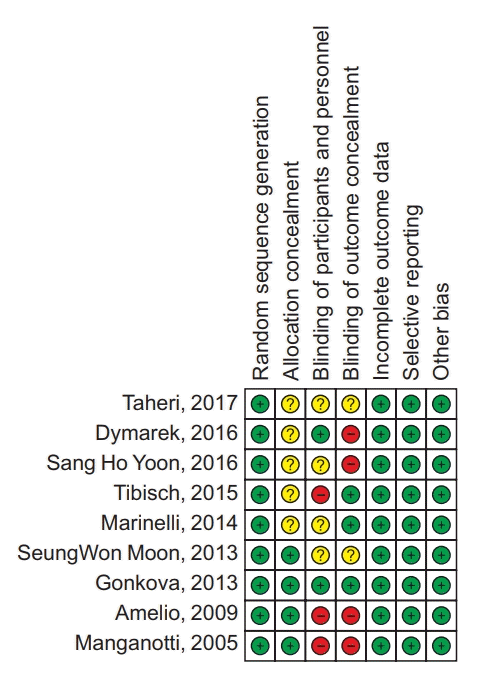
Risk-of-bias assessment
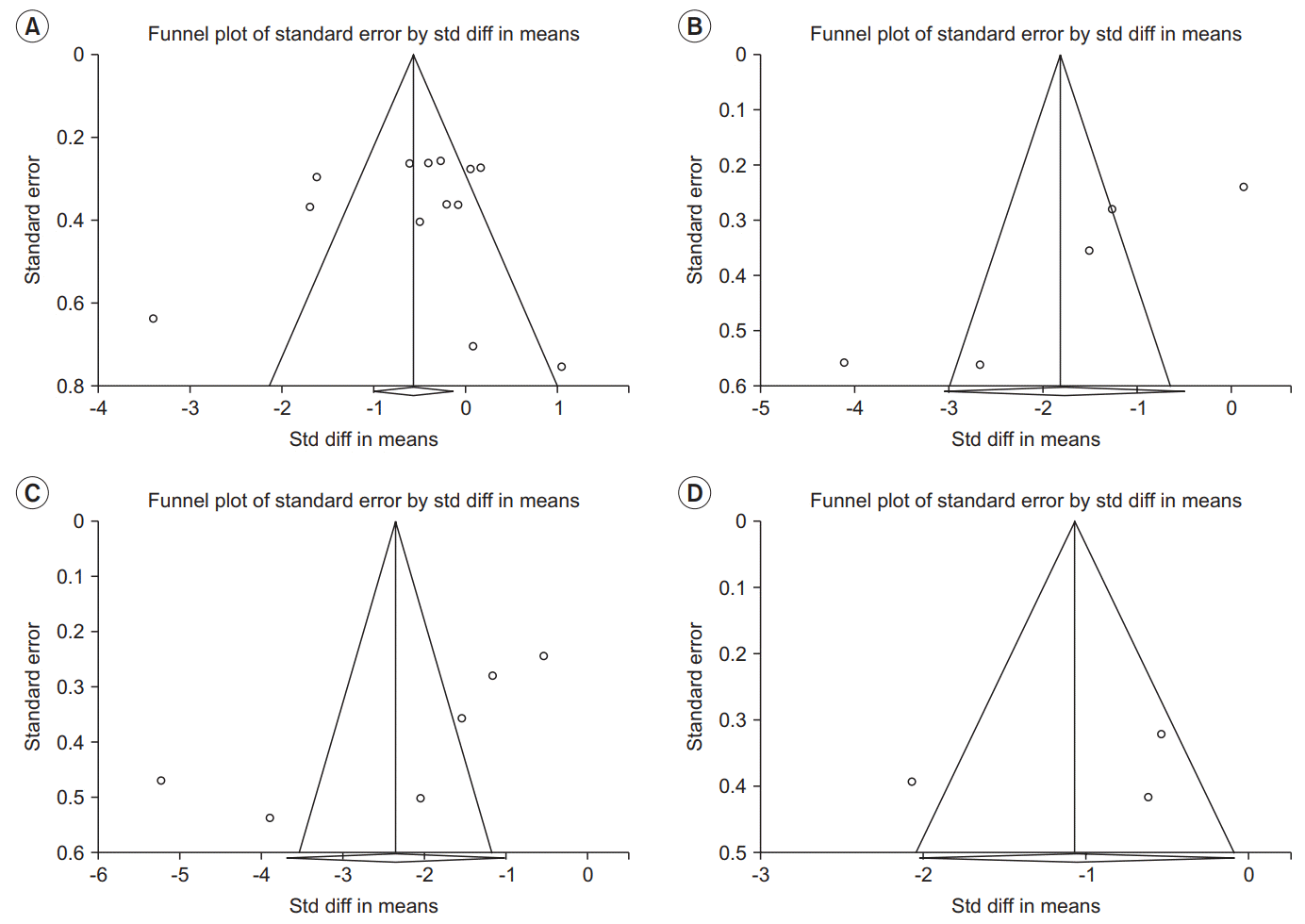
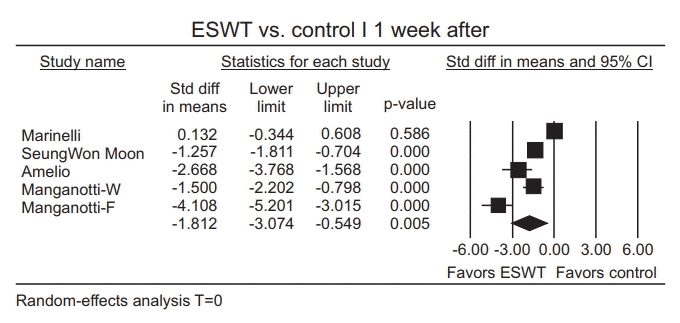
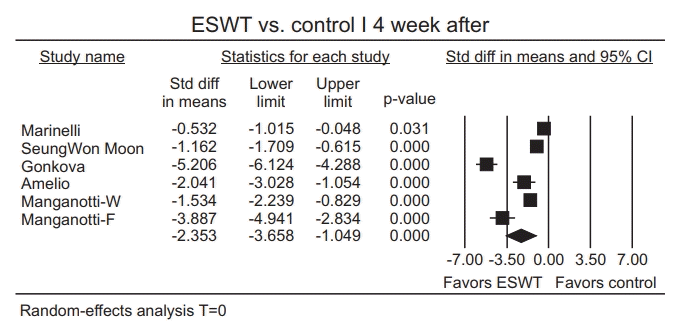
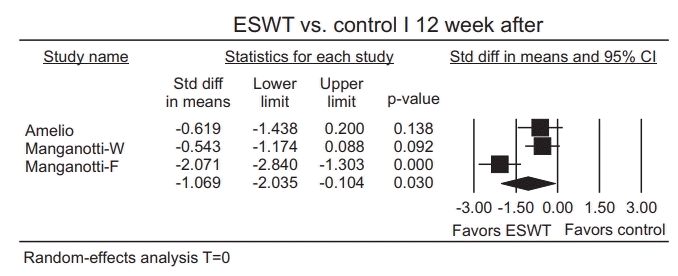
Publication bias
Treatment effects depending on periods
Immediately after ESWT application
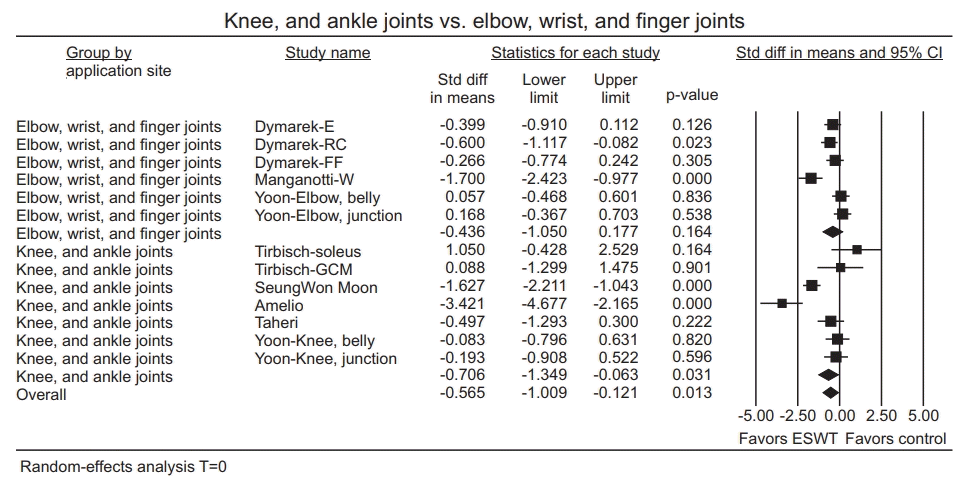
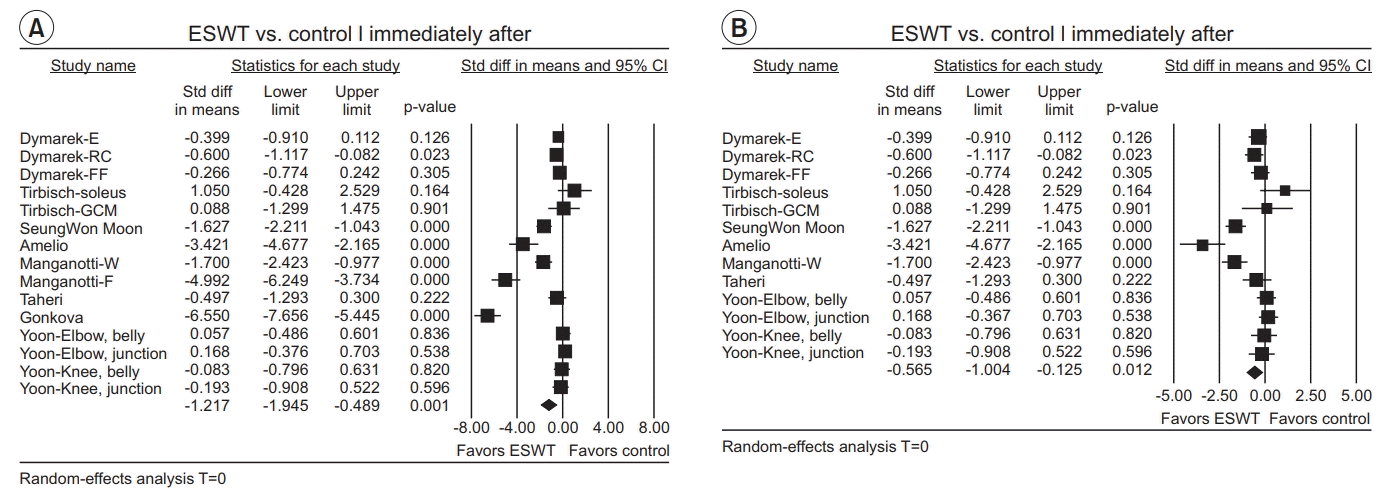 | Fig. 5.Forest plot of effect sizes at (A) immediately after ESWT application, (B) after excluding outliers (Manganotti-F and Gonkova). ESWT, extracorporeal shockwave therapy; Std diff, standard difference; CI, confidence interval; E, elbow joint; RC, radio-carpal joint; FF, finger joints; GCM, gastrocnemius; W, wrist flexors; F, finger flexors. |
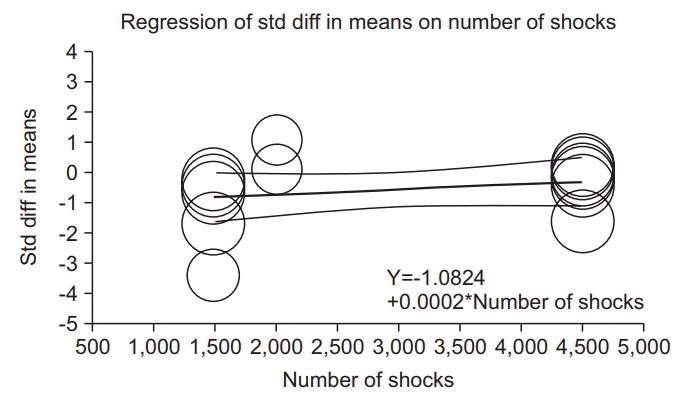
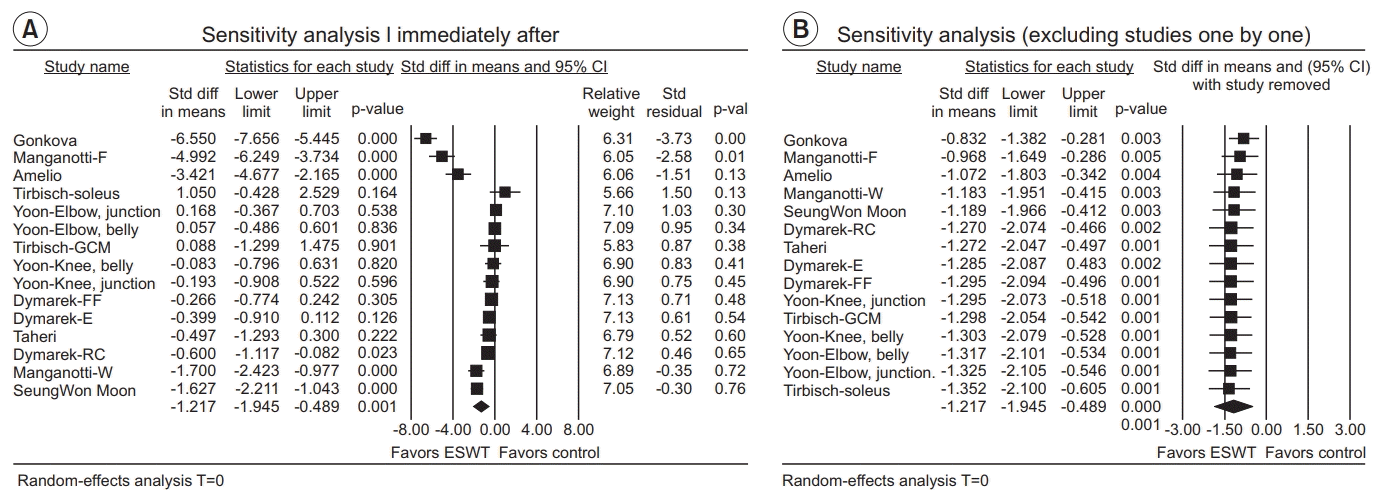 | Fig. 6.(A) Sensitivity analysis with effect sizes, sample size, weight, and residual (immediately after ESWT application). (B) Sensitivity analysis with effect sizes after excluding studies one by one (immediately after ESWT application). ESWT, extracorporeal shockwave therapy; Std diff, standard difference; CI, confidence interval; E, elbow joint; RC, radio-carpal joint; FF, finger joints; GCM, gastrocnemius; W, wrist flexors; F, finger flexors. |
One week after ESWT application
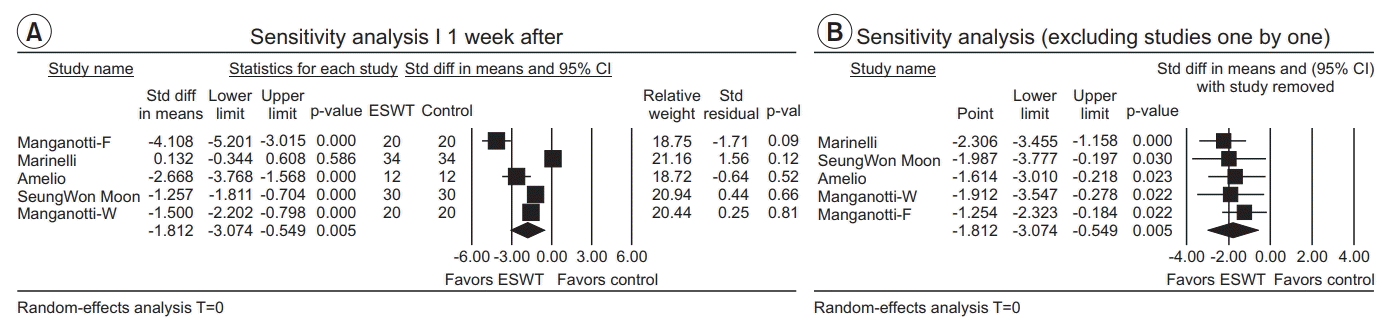 | Fig. 7.(A) Sensitivity analysis with effect sizes, sample size, weight, and residual (1 week after ESWT application). (B) Sensitivity analysis with effect sizes after excluding studies one by one (1 week after ESWT application). ESWT, extracorporeal shockwave therapy; Std diff, standard difference; CI, confidence interval; W, wrist flexors; F, finger flexors. |
Four weeks after ESWT application
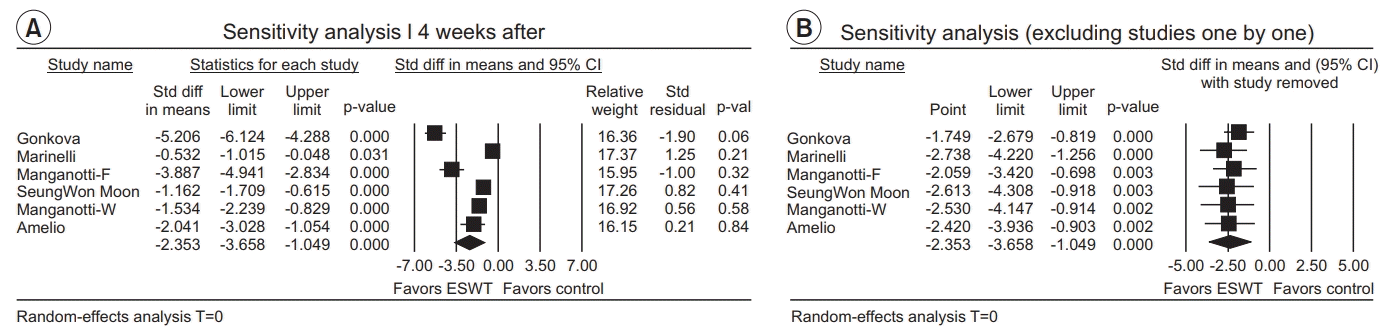 | Fig. 9.(A) Sensitivity analysis with effect sizes, sample size, weight, and residual (4 weeks after the ESWT application). (B) Sensitivity analysis with effect sizes after excluding studies one by one (4 weeks after the ESWT application). ESWT, extracorporeal shockwave therapy; Std diff, standard difference; CI, confidence interval; W, wrist flexors; F, finger flexors. |
Twelve weeks after ESWT application
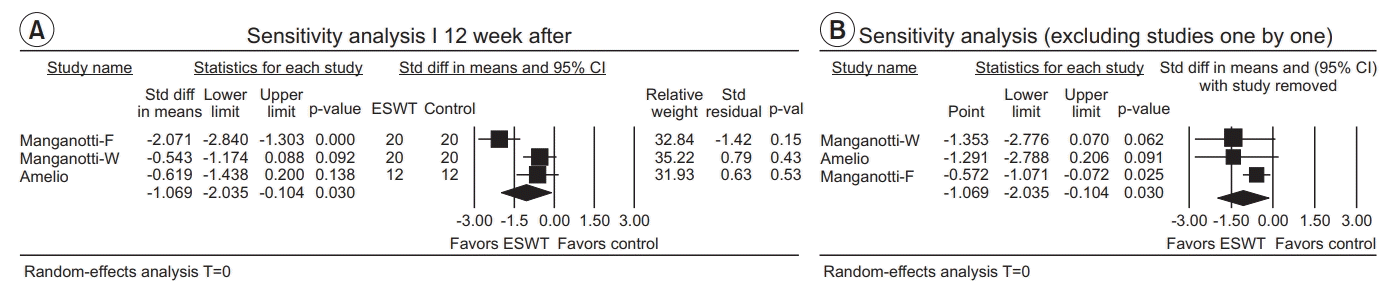 | Fig. 11.(A) Sensitivity analysis with effect sizes, sample size, weight, and residual (12 weeks after ESWT application). (B) Sensitivity analysis with effect sizes after excluding studies one by one (12 weeks after ESWT application). ESWT, extracorporeal shockwave therapy; Std diff, standard difference; CI, confidence interval; W, wrist flexors; F, finger flexors. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download