INTRODUCTION
MATERIALS AND METHODS
Subjects
Table 1.
Table 2.
Treatments
Neurological assessment
Functional assessment
Statistical analysis
RESULTS
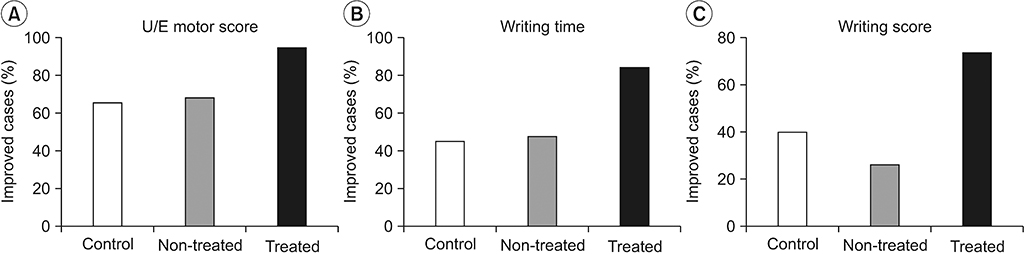 | Fig. 1.Featured neurological and functional changes in control and rTMS-treated patients: number of subjects with improvement (%) with respect to UE motor score (A), writing time (B) and score (C) by JHFT for controls or rTMS-treated patients of treated and non-treated sides during the follow-up period. All p-values were less than 0.05 by likelihood ratio test. rTMS, repetitive transcranial magnetic stimulation; UE, upper extremity; JHFT, Jebsen Hand Function Test. |
Table 3.
|
Initial |
Follow-up |
Gain |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Non-treated | Treated | p-valuea) | Control | Non-treated | Treated | p-valuea) | Control Non-treated | Non-treated | Treated | p-valuea) | |||
| UE | ||||||||||||||
| Motor | 16.55±7.16 | 17.89±6.63 | 17.05±6.21 | 0.819 | 18.40±6.86* | 21.26±5.11* | 21.11±4.32* | 0.202 | 1.85±2.71 | 3.37±3.44 | 4.05±2.00 | 0.049** | ||
| Sensory | ||||||||||||||
| LT | 7.55±1.90 | 7.21±2.39 | 7.11±2.35 | 0.808 | 8.85±1.53 | 7.89±2.45 | 7.74±2.40 | 0.225 | 1.3±1.85 | 0.68±1.67 | 0.63±1.54 | 0.393 | ||
| PP | 7.85±2.13 | 7.42±2.43 | 7.26±2.45 | 0.720 | 8.95±1.54 | 7.89±2.45 | 7.74±2.40 | 0.170 | 1.1±2.03 | 0.47±1.61 | 0.47±1.43 | 0.423 | ||
| Total | 15.40±3.63 | 14.63±4.78 | 14.37±4.75 | 0.749 | 17.80±2.70 | 15.79±4.89 | 15.47±4.80 | 0.184 | 2.4±3.64 | 1.16±3.20 | 1.11±2.88 | 0.378 | ||
| LE | ||||||||||||||
| Motor | 23.50±3.17 | 24.11±2.51 | 22.89±4.47 | 0.604 | 23.50±3.17 | 24.58±1.39 | 24.21±1.99 | 0.376 | 0.23±0.73 | 0.47±1.65 | 1.32±2.98 | 0.145 | ||
| Sensory | ||||||||||||||
| LT | 8.90±1.92 | 7.79±2.49 | 8.32±2.36 | 0.316 | 9.00±2.31 | 8.16±2.48 | 8.42±2.39 | 0.365 | 0.10±0.99 | 0.11±0.46 | 0.11±0.46 | 1.000 | ||
| PP | 8.90±1.86 | 7.89±2.54 | 8.42±2.39 | 0.392 | 8.93±2.61 | 8.16±2.48 | 8.42±2.39 | 0.446 | 0.03±0.48 | 0.00±0.00 | 0.00±0.00 | 0.997 | ||
| Total | 17.80±3.66 | 15.68±5.00 | 16.74±4.72 | 0.345 | 17.93±4.90 | 16.32±4.96 | 16.84±4.78 | 0.405 | 0.13±1.10 | 0.11±0.46 | 0.11±0.46 | 1.000 | ||
| MP (kg) | ||||||||||||||
| GP | 24.38±21.70 | 26.58±34.58 | 18.00±24.52 | 0.607 | 32.83±23.52* | 35.96±35.24* | 32.68±29.80* | 0.931 | 8.45±7.43 | 11.13±9.86 | 13.96±10.63 | 0.202 | ||
| FP | 3.33±3.95 | 3.17±4.32 | 1.99±2.97 | 0.494 | 5.08±6.34* | 4.89±5.14* | 4.53±2.67* | 0.937 | 1.75±2.10 | 1.87±2.53 | 2.43±2.67 | 0.667 | ||
| LP | 5.05±6.34 | 5.96±8.06 | 4.04±6.07 | 0.690 | 7.30±7.51* | 7.94±6.63* | 7.44±6.52* | 0.957 | 2.25±4.79 | 2.48±4.45 | 3.29±3.09 | 0.730 | ||
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; Non-treated, non-treated side of patients who received rTMS; Treated, treated side of patients who received rTMS; UE, upper extremities; LT, light touch; PP, pin prick; LE, lower extremities; MP, muscle power; GP, grasp power; FP, fingertip pinch; LP, lateral pinch.
Table 4.
|
Initial |
Follow-up |
Gain |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Non-treated | Treated | p-valuea) | Control | Non-treated | Treated | p-valuea) | Control Non-treated | Non-treated | Treated | p-valuea) | |||
| JHFT | ||||||||||||||
| Time | ||||||||||||||
| WT | 45.23±35.22 | 40.02±6.63 | 58.19±41.61 | 0.299 | 37.52±16.97 | 35.94±29.5 | 41.22±32.99* | 0.807 | -7.71±25.96 | -4.09±10.05 | -16.97±27.58 | 0.213 | ||
| CT | 14.79±11.52 | 14.59±8.46 | 14.74±8.24 | 0.988 | 11.89±4.43 | 11.90±7.38* | 12.07±7.33* | 0.995 | -2.90±9.31 | -2.69±4.88 | -2.67±4.7 | 0.993 | ||
| SCO | 18.18±7.67 | 18.28±10.15 | 18.97±9.36 | 0.958 | 15.16±7.68* | 15.39±9.74* | 15.93±9.99* | 0.965 | -3.02±5.27 | -2.89±3.54 | -3.04±2.97 | 0.992 | ||
| SF | 19.92±9.22 | 19.70±11.86 | 23.31±12.86 | 0.550 | 15.69±9.14* | 15.56±10.02* | 17.92±12.82* | 0.747 | -4.23±10.57 | -4.15±5.71 | -5.39±7.39 | 0.873 | ||
| SC | 13.55±7.62 | 12.50±9.13 | 13.06±9.65 | 0.993 | 10.32±6.1 | 9.66±*.42* | 10.74±8.66* | 0.912 | -3.23±4.78 | -2.84±4.23 | -2.32±4.79 | 0.825 | ||
| LLO | 8.38±3.68 | 7.19±3.27 | 8.20±3.63 | 0.533 | 7.09±3.70* | 7.03±5.12 | 7.07±4.48* | 0.999 | -1.29±2.37 | -0.15±3.23 | -1.12±3.31 | 0.448 | ||
| LHO | 11.66±6.88 | 11.31±8.22 | 12.34±8.86 | 0.921 | 8.67±5.96* | 7.60±5.91* | 8.01±6.19* | 0.855 | -2.99±4 | -3.70±4.96 | -4.33±6.5 | 0.726 | ||
| Score | ||||||||||||||
| WT | 7.40±4.95 | 9.05±5.89 | 7.16±5.88 | 0.525 | 9.78±4.68* | 9.84±5.17 | 10.21±5.42* | 0.960 | 2.38±3.69 | 0.79±2.02 | 3.05±3.44 | 0.049** | ||
| CT | 2.23±2.41 | 2.74±4.03 | 2.16±3.61 | 0.848 | 3.95±3.90* | 4.00±5.62 | 4.05±5.54* | 0.998 | 1.73±2.73 | 1.26±3.54 | 1.89±3.23 | 0.818 | ||
| SCO | 4.60±4.71 | 5.89±5.41 | 4.37±5.12 | 0.609 | 7.25±6.44* | 7.47±5.52* | 7.32±5.37* | 0.992 | 2.65±3.61 | 1.58±2.22 | 2.95±3.17 | 0.357 | ||
| SF | 6.78±5.73 | 7.63±6.25 | 5.79±6.21 | 0.646 | 8.88±5.82* | 9.32±5.79* | 8.63±6.45* | 0.939 | 2.10±3.29 | 1.68±2.69 | 2.84±3.52 | 0.529 | ||
| SC | 3.95±4.12 | 5.95±5.45 | 5.63±5.59 | 0.422 | 6.53±5.04* | 7.84±5.52* | 6.53±5.80* | 0.691 | 2.58±3.32 | 1.89±3.81 | 0.89±2.4 | 0.274 | ||
| LLO | 7.30±4.25 | 7.26±5.43 | 5.84±5.73 | 0.611 | 9.15±4.61* | 9.16±5.48* | 8.58±5.12* | 0.921 | 1.85±3.18 | 1.89±2.87 | 2.74±3.46 | 0.625 | ||
| LHO | 5.33±4.47 | 5.63±5.27 | 5.32±5.72 | 0.977 | 8.23±5.24* | 8.42±5.38* | 7.74±5.27* | 0.919 | 2.90±3.74 | 2.79±3.33 | 2.42±3.49 | 0.907 | ||
| Total | 36.38±26.79 | 43.95±32.76 | 36.26±35.22 | 0.693 | 53.68±28.37* | 56.11±34.40* | 53.05±34.62* | 0.954 | 17.30±12.04 | 12.16±9.87 | 16.79±12.76 | 0.327 | ||
| OFDT | ||||||||||||||
| 1st half | 467.86±171.53 | 476.04±118.98 | 481.56±129.30 | 0.955 | 401.13±167.91* | 396.40±159.73* | 427.96±166.69* | 0.852 | -66.74±74.34 | -79.64±88.02 | -53.61±63.90 | 0.660 | ||
| 2nd half | 471.37±182.93 | 465.89±127.61 | 472.53±137.39 | 0.990 | 421.79±172.30* | 383.99±166.96* | 417.51±174.26* | 0.765 | -49.58±73.61 | -81.90±86.44 | -55.02±65.47 | 0.392 | ||
| RS | 477.01±166.06 | 483.16±117.55 | 486.46±126.34 | 0.977 | 423.46±159.57* | 400.91±161.69* | 431.05±165.26* | 0.864 | -53.55±79.8 | -82.25±93.31 | -55.41±67.59 | 0.516 | ||
| SS | 1.98±2.02 | 2.11±1.92 | 1.92±1.89 | 0.956 | 3.00±2.43* | 3.03±2.36* | 2.50±2.38* | 0.746 | 1.03±1.81 | 0.92±1.02 | 0.58±0.75 | 0.534 | ||
| PR | 9.87±22.01 | 10.02±21.99 | 8.30±15.18 | 0.958 | 20.08±29.21* | 25.52±33.50* | 19.52±28.08* | 0.813 | 10.21±17.72 | 15.50±24.93 | 11.22±17.79 | 0.695 | ||
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; Non-treated, non-treated side of patients who received rTMS; Treated, treated side of patients who received rTMS; JHFT, Jebsen Hand Function Test; WT, writing; CT, card turning; SCO, picking up small common objects; SF, simulated feeding; SC, stacking checkers; LLO, picking up large light objects; LHO, picking up large heavy objects; OFDT, O’Connor Finger Dexterity Test; RS, raw score; SS, standard score; PR, percentile rank.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download