Abstract
Purpose
The purpose of this study is to present our preliminary experience of the temporary endovascular bypass (TEB) technique using an Enterprise stent for recanalization of acute intracranial artery (IA) occlusion.
Materials and Methods
Patients treated by TEB were enrolled in this retrospective study from January 2009 to May 2010. All the procedures consist of temporary partial deployment and subsequent retrieval of Enterprise stent, supplemented by intra-arterial infusion of urokinase (UK) and/or tirofiban. According to the thrombolysis in cerebral infarction (TICI) classification, recanalization was evaluated with initial and postprocedural angiography. Safety was evaluated related to the procedure and clinical outcomes were assessed by National Institute of Health Stroke Scale (NIHSS) score at discharge and modified Rankin scale (mRS) score at 3 months.
Results
Eleven patients (median NIHSS 12.8, mean age 61.6 years, male: female = 8:3) with acute IA occlusion were treated with TEB. All the patients presented with TICI 0, and the occluded vessel was the middle cerebral artery (n=7), the basilar artery (n=1), and the distal ICA occlusion (n = 3). IV infusion of tissue plasminogen activator (tPA) was done in 4 patients and mechanical thrombolysis with intra-arterial UK was performed in 9. Recanalization was achieved in 73% (8 patients; TICI ≥ 2). There were no procedure-related complications except for two asymptomatic intracranial hemorrhages. Improvement (≥ 4 points on the NIHSS) and good outcome (mRS ≤2) after 90 days was shown in six patients (55%). One patient died 6 days after procedure.
Since the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial presented the milestone of intra-arterial thrombolysis (IAT), the Interventional Management of Stroke Study (IMS) - II pilot study has proven to be to the advantage of IAT, with a favorable functional outcome [1, 2]. Along with the advance of materials and device technologies, various mechanical approaches have been introduced in the treatment of acute ischemic stroke, such as the Merci mechanical clot retriever (Concentric Medical) or Penumbra (Penumbra Inc). Using this device showed better results for recanalization than the PROACT II trial [3]. However, it is also true that almost 30% of the patients show no response to the currently approved thrombolytic therapy. Recently, several cases series have reported an excellent outcome of recanalization with stent implantation as a rescue therapy for treatment of acute ischemic stroke [4-7]. However, treatment-related issues always exist, which include hemorrhagic complications related to stent implantation, concerns about in-stent thrombosis or stenosis, and stent durability.
The Enterprise stent is a self-expandable, retrievable stent which is currently approved as humanitarian device exemption by the US Food and Drug Administration for use in stent-assisted coil embolization of intracranial wide-neck aneurysm. Although in an off-label practice, these vascular reconstructive stents have been applied with "temporary endovascular bypass (TEB)" technique, which is a revascularization technique using self-expandable stent (SES) for acute stroke refractory to conventional thrombolytic therapy, and some case reports have been subsequently released with a good clinical outcome and fewer complications [8-11]. Also other comparable studies using the Solitaire stent (eV3, Irvine, CA, USA) have shown a remarkable recanalization rate and clinical outcome without stent implantation [12-18].
Therefore, we present our preliminary experience of the TEB technique as a treatment option for acute ischemic stroke.
The institutional review board approval approved our study for a retrospective review of the device used in an off-label application and written informed consent was obtained from the patients' next of kin. The cases of all patients treated by TEB for acute stroke from January 2009 until May 2010 were retrospectively analyzed. Clinical data were collected from our stroke database.
Inclusion criteria for TEB were as follows: 1) clinical diagnosis of acute stroke by neurologist, 2) baseline NIHSS score ≥ 8, except for isolated aphasia, hemianopia, 3) exclusion of intracranial hemorrhage (ICH) by CT or MRI, 4) conf irmation of vessel occlusion by angiography, correlating to neurologic deficit, 5) no clinical or laboratory contraindication for IAT, 6) initiation of treatment within 6 hours of symptom onset for hemispheric stroke and within 9 hours for vertebrobasilar stroke, 7) informed consent by patients' relatives. Exclusion criteria for this study were 1) patients with stroke ≥ 80 years, and 2) patients recanalized after stent implantation. The baseline study consisted of a neurological and physical examination, assessment of stroke severity by the NIHSS, routine blood analysis and electrocardiogram (ECG).
The patient was moved into the angiographic suite and diagnostic angiography was performed to evaluate the occlusive segment of the intracranial arteries (IA) and collateral flows. Under local anesthesia, a 6 Fr Envoy guiding catheter (Codman Neurovascular, Miami Lakes, FL, USA) was advanced to the cervical portion of the involved IA or the vertebral artery, via the right femoral artery. A Prowler select plus microcatheter (Codman Neurovascular) was manipulated over an Agility 0.010 microwire (Codman Neurovascular) to the occlusion segment, confirming the proximal and distal ends of the occluded IA to measure the length of the occlusion segment. In the case of IAT with thrombolytic, 100,000-200,000 units of intra-arterial UK were infused directly into the thrombus via this microcatheter and aggressive mechanical thrombolysis (MT) was performed simultaneously with repeated penetration of the microcatheter and microwire across the thrombus [19]. After verifying lack of success in achieving recanalization for 10-20 minutes, an intracranial self-expandable stent (Enterprise vascular reconstruction device; Codman Neurovascular) with double the length of the occlusion segment was delivered across the occlusion segment via the former microcatheter (Prowler select plus microcatheter). After the utmost length of the retrievable stent was partially deployed across the occluded segment, control angiography was performed to confirm immediate recanalization of the occluded IA and the second microcatheter (Prowler 14 microcatheter; Codman Neurovascular or SL-10 microcatheter; Boston Scientific, USA) was advanced into the proximal end of the occluded IA. An additional 100,000 units of UK and/or tirofiban (less than 1.5 mg) were infused intra-arterially through the second microcatheter in order to dissolve the residual thrombus or prevent formation of an acute in-stent thrombus. A delayed angiography after 30 minutes was performed to maintain recanalization of the temporary stented IA and the stent was resheathed and retrieved carefully. A postprocedural angiography was also done to validate recanalization. If reocclusion of the recanalized IA was shown on the angiography, the second trial of TEB was performed in the reoccluded IA. This procedure was repeated several times until recanalization was obtained. Control angiography was performed to evaluate recanalization of the IA immediately after all procedures. A closure device (Angio-seal; St. Jude medical, St. Paul, MN, USA) was used in all patients for hemostasis at the femoral artery puncture site.
The feasibility of TEB was evaluated by angiographic result of recanalization. The initial and postprocedural angiographies were compared by two neuroradiologists. The status of vessel recanalization was evaluated according to the Thrombolysis in Cerebral Ischemia (TICI) scale[20, 21] (grade 0, no perfusion; grade I, penetration but not perfusion; grade IIa, partial perfusion with incomplete distal filling of < 50% of the expected territory; grade IIb, partial perfusion with incomplete distal filling of 50-99% of the expected territory; grade IIc, near complete perfusion but with delay in contrast runoff; grade III, full perfusion with normal filling of distal branches in a normal hemodynamic fashion) and recanalization after procedure was defined as TICI grades II or III.
The safety of this procedure was evaluated by presence of procedure-related complications, such as vessel dissection/perforation, inguinal hematoma, and postprocedural hemorrhagic event (ICH), either symptomatic (defined as worsening more than 4 points of NIHSS score) or asymptomatic neurologic deterioration.
Clinical outcome was evaluated by the NIHSS score at discharge and the modified Rankin scale (mRS) score at 3-month, which was determined by a neurologist (KYL). A CT was taken 1 day after the procedure for evaluation of ICH and a brain MRI and MR Angiography were performed after 7 days to evaluate the patency of the recanalized vessel and extent of the infarction.
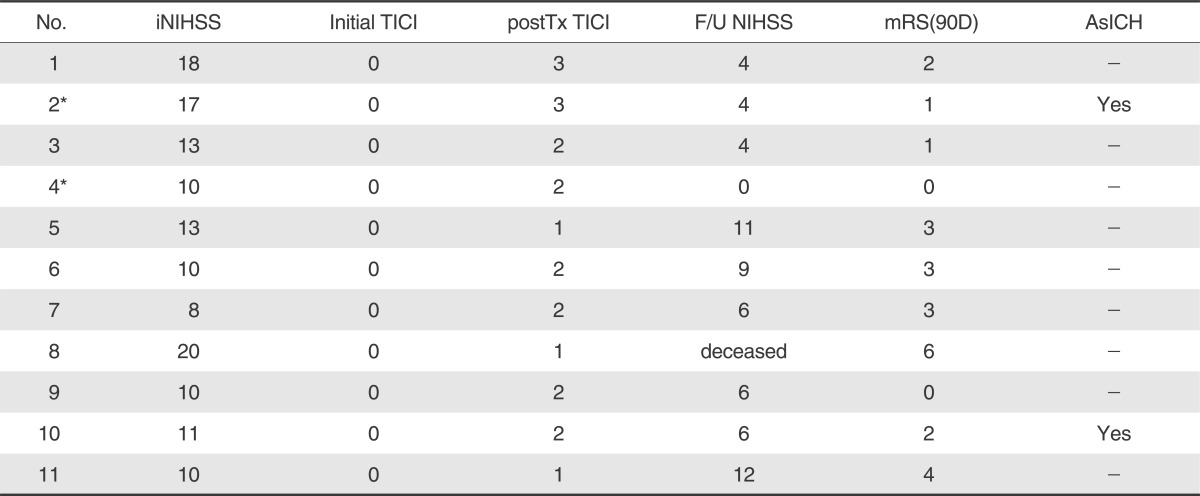
Eleven patients (8 men and 3 women, mean age 61.6±9 years, range 50 to 74) were enrolled in this study from January 2009 to May 2010 (Table 1). The mean NIHSS score at admission was 12.8±4.3 (median 12). All involved vessels presented with TICI 0 and the occluded vessels were the middle cerebral artery (n=7), the basilar artery (n=1), and the distal ICA occlusion (n=3). A brain MRI (n=6) or brain computed tomography angiography (n=10) was performed before procedure [5]. patients had either a previous history of atrial fibrillation or confirmed by ECG on admission and two patients had gastrointestinal malignancies with chemotherapy. Intravenous (IV) tPA (the dose 0.9 mg/kg) was infused in four patients and mechanical thrombolysis with intra-arterial urokinase was performed in nine. TEB was applied as a primary treatment tool in two patients, who had bleeding tendency with malignancy or arrived at hospital just before the golden time of six hours. Intra-arterial tirofiban (less than 1.5 mg) was administered in all patients. One patient with MCA occlusion (patient 2) had concomitant carotid artery stenosis and a stent was placed in the proximal carotid artery before the thrombolytic procedure. Recanalization using TEB was achieved in 8 patients (73%); 8 (73%) had TICI 2 or 3 and 3 had TICI 1(Table 2). There was no periprocedural complication in 11 patients. Mean duration from onset to treatment start time was 235±66 minutes (median 235 minutes, range 124-340 minutes) and mean time from puncture to vessel recanalization was 123±49 minutes (median, 135 minutes; range 50 to 180 minutes). The average frequency of pass attempt was 2.8±0.8 times (median 3times, range 2 to 4 times). 6 patients (55%) improved after the procedure by more than 4 NIHSS points during hospitalization. No patients had symptomatic ICH or worsened neurologic examination; but 5 patients showed no improvement in NIHSS of > 4 points. Two patients (18%) had asymptomatic ICH. A follow-up brain MRI showed preservation of the recanalized vessel 7 days after thrombolysis in 8 patients (73%). The mean NIHSS score at discharge was 6.2±3.8 (median, 6; range 0 to 12). At the 3-month follow-up, mRS of ≤ 2 in 6 patients (55%). No ischemic event or progression of neurological deficit occurred during follow-up. The mortality rate was 9% (1/11; patient 8); After failure of intraarterial(IA) thrombolysis with UK, TEB was repeated 3 times until the golden time of 6 hours but the MCA was not recanalized in the end.
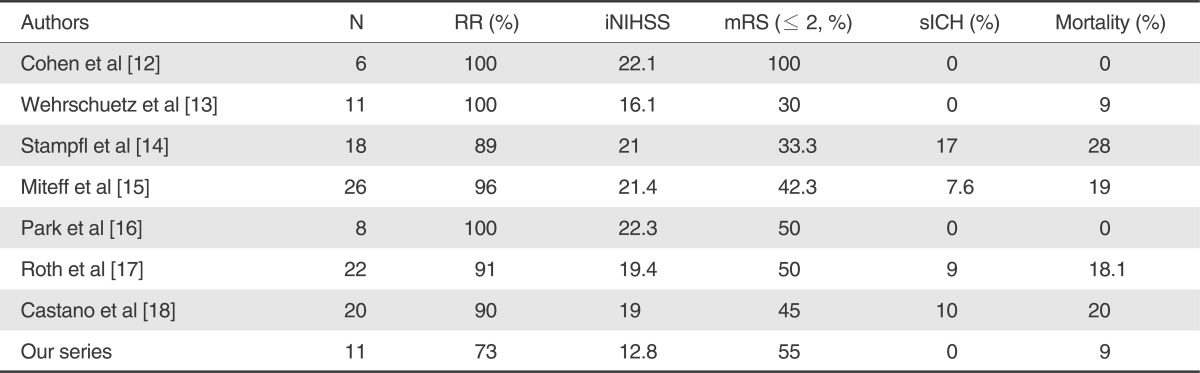
In acute ischemic stroke, rapid recanalization is strongly correlated with good clinical outcome and reduced mortality [22]. While previously published data about recanalization using IAT with thrombolytic (66%), MT with Merci device (69% in TIMI II/III) or the Penumbra system (more than 80%) showed low functional outcome (mRS ≤2 at 90-day ; 28 ~40%), recanalization with stent implantation has recently emerged as a new alternative method, which demonstrates better results for recanalization (79 ~ 100%) and clinical outcome in the treatment of the acute ischemic stroke [1, 3, 4, 6, 23-25]. Although the latter is fairly attractive in terms of rapid recanalization, it has some drawbacks in practice. Particularly, it is a burden to the clinicians that dual antiplatelet regimens need to be administered after stent implantation despite the risk of ICH and that regular follow up is mandatory to avoid delayed complications, such as in-stent restenosis/thrombosis. Recently several authors reported excellent recanalization (more than 90%) and functional outcome (30-100%) was achieved in acute ischemic stroke using mechanical thrombectomy with full-retrievable stent [12-18].
Our study demonstrated that 8 of 11 (73%) patients treated by TEB achieved recanalization of TICI grade 2 or 3 after adjunctive therapy. 6 patients (55%) showed good clinical outcome (mRS≤2 at 90-day) and an improvement of NIHSS≥4 in 6 patients(55%) at discharge. These results showed a favorable procedural and clinical outcome, although the other comparable study using retrievable stents (Table 3) showed 88-100% of recanalization rate and 30-100% of a good functional outcome (mRS≤2 at 90-day) [12-18]. Remarkably, all occluded IAs showed immediate recanalization (TICI ≥ 2) during TEB, and the antegrade arterial flow was maintained with intraarterial infusion of tirofiban (mean dose 1.3±0.3 mg; 11 patients) or urokinase (less than 200,000 IU; 9 patients), which may accelerate thrombolysis endogenously and pharmacologically with the thrombus entrapped. In using the Enterprise stents, there was no limitation of temporary stenting and immediate flow restoration due to their various lengths from 14 to 37 mm, although the length of the thrombus is a limiting factor in Solitaire stent study [18]. Furthermore mean procedure time from puncture to recanalization was 123±49 minutes, and the average frequency of pass attempt was 2.8±0.8 times in this study. This result was also comparable with the previous MERCI study but not superior to the result of the solitaire stent [3, 6, 12-18].
There was none of complication related with procedure using the Enterprise stent. Procedure-related complications were reported to occur in 6-10% of recanalization procedures with stent implantation and 0% with the Solitaire stent [5, 13-18]. Since the introduction of SES, it is possible to overcome vessel tortuosity, the difficulties of navigation, and stiffness of the device itself. Especially the Enterprise stent is superior to other SESs in navigating tortuous cerebral vessel [6, 7, 24, 27]. But it should be careful that manipulation of the Enterprise stent in TEB may be dangerous to dissection or vessel rupture because of its own properties, such as partial deployment with the flared stent ends, which had widened distally to more than 7 mm, contrary to the Solitaire stent.
The symptomatic ICH rate was 0% and the asymptomatic ICH rate was 2 patients (18%), in whom recanalization had achieved successfully and IV or intraarterial thrombolytics and tirofiban were administered. Hemorrhage may be induced by reperfusion injury after recanalization or the administered medications. Actually we used tirofiban combined with other fibrinolytics under the assumption that Use of tirofiban may be necessary to maintain the microcirculation as well as the reperfusion flow during the procedure and enhance the fibrinolytic response. Although the risk of hemorrhage associated with glycoprotein inhibitor and intraarterial/IV fibrinolytics in acute stroke is controversial, it should be kept in mind that tirofiban could raise the possibility of hemorrhage and it could be a better way to choose other treatment option, such as mechanical thrombectomy using penumbra or solitaire stent. In terms of the symptomatic ICH rate, this study presented a comparable outcome to the similar studies using retrievable stent (7-17%) [12-18] and other intra-arterial procedures, such as the PROACT-II, MERCI and Penumbra pivotal study (8-11%) [1, 25, 28].
In this study, recanalization was not achieved in three patients. The causes of procedural failure were as follows; 1) the possibilities of underlying arterial stenosis according to the angiographic findings during and after TEB, 2) a large amounts of the thrombus burden, and 3) difficulty of resheathing the stent due to tortuosity of the involved arteries. When the arteries were occluded with underlying stenosis or an amount of the thrombus burden, it is better to choose another treatment option for the acute ischemic stroke. Moreover, it may be dangerous to apply TEB technique to the arterial occlusion with vascular tortuosity. Although this SES had excellent navigability, stent deployment and resheathing of the deployed stent are distinct procedures. In retrieval of the Enterprise stent, retrograde sheathing of the microcatheter should be performed simultaneously without moving the halfdeployed stent in order to prevent intimal injury or rupture of vessel. Therefore, stent implantation also should be carefully considered as a substitute for retrieval of the stent if performed in long-segmented occlusion of the distal ICA, the underlying stenoocclussive lesion and the tortuous vascular lesion.
There are some limitations to this study; 1) the relatively small numbers of patients, 2) the retrospective evaluation of data collection and analysis, 3) regional limitations of the device application (the Penumbra system, MERCI device, Pharos intracranial stent or solitaire stent) by the health insurance, 4) the off-label use and the limited indication of the Enterprise stent in recanalization of the acute IA occlusion should be stressed and finally, we should state that two of the patients in this study were previously mentioned in an article describing temporary recanalization in the treatment of acute ischemic stroke [10, 11].
In conclusion, TEB may be a valuable treatment option for the treatment of acute thromboembolic IA occlusion without stent implantation. However this technique should be carefully considered in case of the suspicious steno-occlusive lesion, anatomical difficulty and occlusion with a large thrombus burden.
Acknowledgement
This work was supported (in part) by grant from the Korean Stroke Society young investigator's award (KSS 2010-003)
References
1. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The proact II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999; 282:2003–2011. PMID: 10591382.
2. Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol. 2008; 29:582–587. PMID: 18337393.

3. Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008; 39:1205–1212. PMID: 18309168.
4. Levy EI, Mehta R, Gupta R, Hanel RA, Chamczuk AJ, Fiorella D, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007; 28:816–822. PMID: 17494649.
5. Zaidat OO, Wolfe T, Hussain SI, Lynch JR, Gupta R, Delap J, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke. 2008; 39:2392–2395. PMID: 18556584.

6. Brekenfeld C, Schroth G, Mattle HP, Do DD, Remonda L, Mordasini P, et al. Stent placement in acute cerebral artery occlusion: use of a self-expandable intracranial stent for acute stroke treatment. Stroke. 2009; 40:847–852. PMID: 19182080.
7. Mocco J, Hanel RA, Sharma J, Hauck EF, Snyder KV, Natarajan SK, et al. Use of a vascular reconstruction device to salvage acute ischemic occlusions refractory to traditional endovascular recanalization methods. J Neurosurg. 2010; 112:557–562. PMID: 19764826.

8. Kelly ME, Furlan AJ, Fiorella D. Recanalization of an acute middle cerebral artery occlusion using a self-expanding, reconstrainable, intracranial microstent as a temporary endovascular bypass. Stroke. 2008; 39:1770–1773. PMID: 18388338.

9. Hauck EF, Mocco J, Snyder KV, Levy EI. Temporary endovascular bypass: a novel treatment for acute stroke. AJNR Am J Neuroradiol. 2009; 30:1532–1533. PMID: 19279279.
10. Suh SH, Lee KY, Hong CK, Kim BM, Kim CH, Chung TS, et al. Temporary stenting and retrieval of the self-expandable, intracranial stent in acute middle cerebral artery occlusion. Neuroradiology. 2009; 51:541–544. PMID: 19424689.

11. Suh SH, Kim BM, Roh HG, Lee KY, Park SI, Kim DI, et al. Self-expanding stent for recanalization of acute embolic or dissecting intracranial artery occlusion. AJNR Am J Neuroradiol. 2010; 31:459–463. PMID: 19892814.

12. Cohen JE, Gomori JM, Leker RR, Eichel R, Arkadir D, Itshayek E. Preliminary experience with the use of self-expanding stent as a thrombectomy device in ischemic stroke. Neurol Res. 2011; 33:214–219. PMID: 21801598.

13. Wehrschuetz M, Wehrschuetz E, Augustin M, Niederkorn K, Deutschmann H, Ebner F. Early single center experience with the solitaire thrombectomy device for the treatment of acute ischemic stroke. Interv Neuroradiol. 2011; 17:235–240. PMID: 21696665.

14. Stampfl S, Hartmann M, Ringleb PA, Haehnel S, Bendszus M, Rohde S. Stent placement for flow restoration in acute ischemic stroke: a single-center experience with the solitaire stent system. AJNR Am J Neuroradiol. 2011; 32:1245–1248. PMID: 21596812.
15. Miteff F, Faulder KC, Goh AC, Steinfort BS, Sue C, Harrington TJ. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (solitaire AB): experience in 26 patients with acute cerebral artery occlusion. AJNR Am J Neuroradiol. 2011; 32:1078–1081. PMID: 21493763.
16. Park H, Hwang GJ, Jin SC, Jung CK, Bang JS, Han MK, et al. A retrieval thrombectomy technique with the solitaire stent in a large cerebral artery occlusion. Acta Neurochir (Wien). 2011; 153:1625–1631. PMID: 21479799.

17. Roth C, Papanagiotou P, Behnke S, Walter S, Haass A, Becker C, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke. 2010; 41:2559–2567. PMID: 20947848.

18. Castaño C, Dorado L, Guerrero C, Millán M, Gomis M, Perez de la Ossa N, et al. Mechanical thrombectomy with the solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke. 2010; 41:1836–1840. PMID: 20538693.
19. Yoon W, Park MS, Cho KH. Low-dose intra-arterial urokinase and aggressive mechanical clot disruption for acute ischemic stroke after failure of intravenous thrombolysis. AJNR Am J Neuroradiol. 2010; 31:161–164. PMID: 19713319.

20. Noser EA, Shaltoni HM, Hall CE, Alexandrov AV, Garami Z, Cacayorin ED, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke. 2005; 36:292–296. PMID: 15625300.
21. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003; 34:e109–e137. PMID: 12869717.

22. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007; 38:967–973. PMID: 17272772.
23. The Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009; 40:2761–2768. PMID: 19590057.
24. Levy EI, Siddiqui AH, Crumlish A, Snyder KV, Hauck EF, Fiorella DJ, et al. First food and drug administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke). Stroke. 2009; 40:3552–3556. PMID: 19696415.
25. Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke. 2005; 36:1432–1438. PMID: 15961709.
26. Jovin TG, Gupta R, Uchino K, Jungreis CA, Wechsler LR, Hammer MD, et al. Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke. 2005; 36:2426–2430. PMID: 16224082.

27. Chiam PT, Samuelson RM, Mocco J, Hanel RA, Siddiqui AH, Hopkins LN, et al. Navigability trumps all: stenting of acute middle cerebral artery occlusions with a new self-expandable stent. AJNR Am J Neuroradiol. 2008; 29:1956–1958. PMID: 18768730.

28. Bose A, Henkes H, Alfke K, Reith W, Mayer TE, Berlis A, et al. The penumbra system: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008; 29:1409–1413. PMID: 18499798.

Table 1
Patients' characteristics using TEB technique
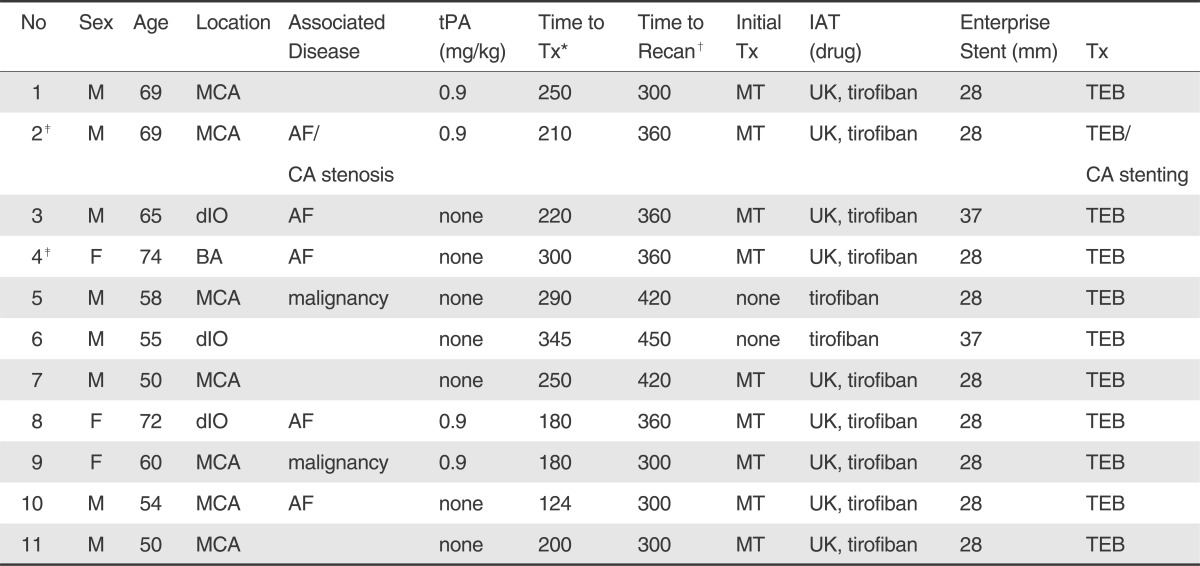
Abbreviations: No, number; M, male; F, female; MCA, middle cerebral artery; BA, basilar artery; dIO, distal intracranial artery occlusion; tPA, tissue plasminogen activator; Tx, treatment; MT, mechanical thrombolysis; IAT, intraarterial thrombolysis; UK, urokinase; ICH, intracranial hemorrhage; NA, not available; AF, atrial fibrillation; CA, carotid artery; TEB, temporary endovascular bypass
*= time from onset to treatment (minutes)
†= time to recanalization (minutes);




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download