Abstract
Development of de novo aneurysm or aneurysm regrowth after complete clipping of an intracranial aneurysm is rare. We report coiled cases of de novo aneurysm and aneurysm regrowth. We retrospectively reviewed 107 cases of intracranial aneurysm coiling performed in our hospital, identifying five cases of coiled aneurysm that were de novo aneurysm or aneurysm regrowth. In all the cases, total or near total occlusion was seen. There were no complications related to the procedure. In two of the three patients with ruptured aneurysms, consciousness level on admission was stupor. When the patient was discharged after the treatment, one of them had 4 of the modified Rankin Scale. The other one had 5 on discharge. The rest three patients had 0. As for a de novo aneurysm or a regrowth of aneurysm, coiling may be considered when clipping is difficult.
Development of de novo aneurysm or aneurysm regrowth after complete clipping of an intracranial aneurysm is rare, with incidence 0.14-2% [1, 2]. Revision surgery in case of the events is difficult due to adhesion of wound and changes in normal anatomical structure. Cosmetic issue and delayed wound healing related to the surgery also concerns of patients. According to recent researches which compared clipping and coiling for patients with cerebral aneurysm, recurrence rate is higher post to coiling but there is no difference in clinical prognosis between them [3, 4]. We report coiled cases of de novo aneurysm and aneurysm regrowth in patients who previously underwent clipping.
Between January 1 and December 31, 2011, we retrospectively reviewed 107 cases of intracranial aneurysm coiling performed in our hospital, identifying five cases of coiled aneurysm that were de novo aneurysm or aneurysm regrowth. One patient was male, and the others were female. The mean follow-up period was 14 years (9-17 years); three aneurysms were ruptured and two aneurysms were unruptured.
There are two middle cerebral artery (MCA) aneurysms, one posterior communicating artery (P-com) aneurysm, one anterior choroidal artery aneurysm, and one basilar top aneurysm. In all the cases, total or nearly total occlusion was seen. There were no complications related to the procedure. In two of the three patients with ruptured aneurysms, consciousness level at admission was stupor. One of them had 1 of modified Rankin Scale (mRS) before the rupture but the score was recorded as 4 when the patient was discharged after treatment; the other one had 3 before the rupture but the score was recorded as 5 when the patient was discharged. The rest three patients had 0 (Table 1).
The patient was a 46-year-old male who underwent clipping 17 years ago because of ruptured basilar top aneurysm. The aneurysm was found by Computed Tomography (CT) angiography during health screening. CT images identified the aneurysm 17 years ago could not be obtained. The patient had a wide neck aneurysm, 7.8 mm in size, with regrowth from the aneurysm neck. The aneurysm regrowth was treated by coiling using double microcatheter technique. Two microcatheters (Echelon-10, EV3, Irvine, CA, USA) were introduced into the basilar top aneurysm through both vertebral arteries. Ten coils were used for the aneurysm out of 11 coils (total 40 centimeters), resulting near total occlusion. There was no difficulty in coiling the aneurysm through clip shadow. The patient was discharged with 0 of mRS.
The patient was a 61-year-old female who underwent clipping 17 years ago because of ruptured right MCA (M1) aneurysm. The aneurysm was found by CT angiography during health screening. CT images identified the aneurysm 17 years ago could not be obtained. The patient had a saccular aneurysm, 3.2 mm in size, with regrowth from the aneurysm dome. The aneurysm regrowth was treated by coiling using single microcatheter technique. One microcatheter (Echelon-10) was introduced into MCA aneurysm. Five coils were required for the aneurysm (total 12 centimeters), indicating near total occlusion. There was no difficulty in coiling the aneurysm through clip shadow. The patient was discharged with 0 of mRS.
The patient was a 47-year-old female who underwent clipping because of right MCA bifurcation aneurysm ruptured 14 years ago. She visited our hospital with recurrent subarachnoid hemorrhage (SAH). On visiting, her mRS was 1 and Hunt and Hess grade was 2. Images of diagnostic studies identified the aneurysm 14 years ago could not be obtained. The patient had a saccular aneurysm, 4.8 mm in size, with regrowth from the aneurysm neck. The aneurysm regrowth was treated by coiling using single microcatheter technique. One microcatheter (Echelon-10) was introduced into MCA aneurysm. Three coils were required for the aneurysm (total 13 centimeters), indicating near total occlusion. There was no difficulty in coiling the aneurysm through clip shadow. The patient was discharged with 0 of mRS.
A 72-yer-old female patient underwent clipping because of left P-com aneurysm ruptured 9 years ago. She had a history of recurrent SAH and was also treated for cerebral infarction in posterior inferior cerebellar artery territory 2 years ago. Her mRS was 3 before the aneurysm rupture. Consciousness level on admission was stupor. Her modified Fisher Scale was 4 and Hunt and Hess grade was 4. She had a de novo aneurysm originated from left anterior choroidal artery. Images of diagnostic studies identified the aneurysm 9 years ago could not be obtained. CT angiography was carried out two years ago which suspected of aneurysmal dilatation. However, further evaluation was not performed due to her old age. She had a saccular wide neck aneurysm, 6 mm in size. Coiling with single microcatheter technique was performed for the patient. One microcatheter (Echelon-10) was introduced into aneurysm. 4 coils were used for the aneurysm (total 22 centimeters), indicating near total occlusion. There was no difficulty in coiling the aneurysm through clip shadow. Her mRS was 5 when she was discharged.
The patient was a 66-year-old female who underwent clipping because of right P-com aneurysm ruptured 13 years ago. Last year, the patient underwent ventricular-peritoneal shunt and treated due to cerebral infarction. She visited our hospital with recurrent SAH. She had aneurysm regrowth, and her modified Fisher Scale and Hunt and Hess grade were 4, respectively. Consciousness level on admission was stupor. There was saccular wide neck aneurysm, 8 mm in size. Images of diagnostic studies identified the aneurysm 13 years ago could not be obtained. Coiling with double microcatheter technique was performed for the patient. Two microcatheters (Echelon-10) were introduced into the aneurysm. 15 coils were used for the aneurysm (total 116 centimeters), indicating near total occlusion. There was no difficulty in coiling the aneurysm through clip shadow. Her mRS was 1 before the aneurysm rupture and was 4 when she was discharged.
SAH after surgical clipping is generally considered as a rare event, however, recurrent SAH in patients of SAH clipping is rather frequently found. Literature review identified that the incidence is reported to be 0.14% to 2% [1, 2]. Tsutsumi et al. [5] reported that recurrence of SAH was at least 10 folds higher in patients who underwent SAH clipping than general population and the recurrence rate was similar in patients who had surgical treatment for unruptured aneurysms. Recurrent SAH after clipping is caused by rupture of a recurrent aneurysm at the clip site or by rupture of an untreated additional or a de novo aneurysm.
Several mechanisms are involved in development of recurrent aneurysms. First, there may be a remaining defect of the internal elastic lamina causing aneurysm formation leading to de novo aneurysm although ruptured aneurysms is successfully treated in some cases. Second, aneurysms may be missed during the first treatment due to limited sensitivity of angiography. Third, regrowth of an aneurysm may be formed by clip slippage which may be induced by the wide and partially calcified aneurysmal neck, grasping of the aneurysmal neck with the distal third of the clip blades, or magnetic resonance image investigation [1]. Fourth, it is also possible that recurrent aneurysms may be caused by incomplete clipping [6].
Byrne et al. [7] reported that the risk of regrowth from completely clipped aneurysms (0.26%, annually) was much lower than the risk of de novo aneurysm formation (0.89%, annually). Several researchers reported that the annual incidence of de novo aneurysm was between 0.37 and 1.20% [2, 8]. The cumulative risk becomes significant after 9 years, and the accumulated risk of aneurysm recurrence may exceed 10%. Therefore, Follow-up study such as angiography or CT angiography may be considered for patients who underwent complete clipping of aneurysms 9 to 10 years before [2]. According to Kim et al. [9], rupture after clipping was the only predictor for poor surgical outcomes. Therefore, it is expected that treating aneurysm before they rupture will improve surgical outcomes and this can be achieved by regular followup study such as angiography, CT angiography or magnetic resorance angiography.
Literature review revealed that young age and small residual neck may serve as risk factors for aneurysm recurrence [10], while hypertension, hemodynamic stress, female patients, young age, previous SAH, smoking, congenital diseases, and family history of cerebrovascular disease may serve as risk factors for de novo aneurysm [11].
Revision surgery can be problematic due to changes in normal anatomical structure, scarring around aneurysms, preplaced clips, coating materials and previous incomplete clipping. Incomplete clipping usually occur in complicated cases. Therefore, previous incomplete clipping suggests that revision surgery will be complicated one just like the first one and there will be a higher risk of procedure-related complications [12].
Although coiling is controversial because it has a relatively higher recurrence rate than clipping and needs additional treatments. However, according to a long-term follow-up study of the ISAT with the mean 9 year follow-up, it was found that there was no difference in mortality caused by rebleeding between the coiling group and the clipping group although the rebleeding rate of the coiling group was higher occurred [3]. Furthermore, in other studies with short-term follow-ups, it was reported that a long-term prognosis and risk of recurrent SAH of patients who were adequately treated by coiling was comparable to those patients who were treated by clipping [4].
In addition, there are increasing numbers of studies reporting favorable outcomes of coiling performed for de novo aneurysm or aneurysm regrowth [13, 14]. These results suggest that coiling can be considered as an alternative treatment option to clipping in the retreatment of intracranial aneurysm of de novo formation or regrowth.
In conclusion, coiling may be considered when clipping is infeasible for a de novo aneurysm or a regrowth of aneurysm. Incidence of de novo formation or regrowth is related to follow-up duration and follow-up image study after treatment of aneurysm is worth consideration.
References
1. Asgari S, Wanke I, Schoch B, Stolke D. Recurrent hemorrhage after initially complete occlusion of intracranial aneurysms. Neurosurg Rev. 2003; 26:269–274. PMID: 12802695.

2. Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms : results of long-term follow-up angiography. Stroke. 2001; 32:1191–1194. PMID: 11340232.
3. Molyneux AJ, Kerra RSC, Birksb J, Ramzia N, Yarnolda J. ISAT collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009; 8:427–433. PMID: 19329361.

4. Schaafsma JD, Sprengers ME, van Rooij WJ, Sluzewski M, Majoie CB, Wermer MJ, et al. Long-Term recurrent subarachnoid hemorrhage after adequate coiling versus clipping of ruptured intracranial aneurysms. Stroke. 2009; 40:1758–1763. PMID: 19286603.

5. Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of subarachnoid hemorrhage after surgical treatment of unruptured cerebral aneurysms. Stroke. 1999; 30:1181–1184. PMID: 10356096.

6. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008; 39:120–125. PMID: 18048860.
7. Byrne JV, Sohn M, Molyneux AJ. Five-year experience in using coil Embolization for ruptured intracranial aneurysms: outcomes and Incidence of late rebleeding. J Neurosurg. 1999; 90:656–663. PMID: 10193610.

8. Wermer MJ, vander Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ. ASTRA Study Group. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005; 128(Pt 10):2421–2429. PMID: 16000333.

9. Kim BM, Kim DJ, Kim DI, Park SI, Suh SH, Won YS. Clinical presentation and outcomes of coil embolization of remnant or recurred intracranial aneurysm after clipping. Neurosurgery. 2010; 66:1128–1133. PMID: 20495427.

10. Nakase H, Kamada Y, Aoki H, Goda K, Morimoto T, Sasaki T. Clinical study on recurrent intracranial aneurysm. Cerebrovasc Dis. 2000; 10:255–260. PMID: 10878429.
11. Tonn J, Hoffmann O, Hofmann E, Schlake HP, Sörensen N, Roosen K. "De novo" formation of intracranial aneurysms: who is at risk? Neuroradiology. 1999; 41:674–679. PMID: 10525770.

12. Wermer MJ, Rinkel GJ, Greebe P, Albrecht KW, Dirven CM, Tulleken CA. Late recurrence of subarachnoid hemorrhage after treatment for ruptured aneurysms: patient characteristics and outcomes. Neurosurgery. 2005; 56:197–204. PMID: 15670367.

13. Bendok BR, Ali MJ, Malisch TW, Russell EJ, Batjer HH. Coiling of cerebral aneurysm remnants after clipping. Neurosurgery. 2002; 51:693–697. PMID: 12188947.

14. Forsting M, Albert FK, Jansen O, von Kummer R, Aschoff A, Kunze S, et al. Coil placement after clipping: endovascular treatment of incompletely clipped aneurysms. Report of two cases. J Neurosurg. 1996; 85:966–969. PMID: 8893741.
Fig. 1
Cerebral angiography reveals regrowth of basilar top aneurysm (arrow) (A). Cerebral angiography shows nearly total obliteration (B).
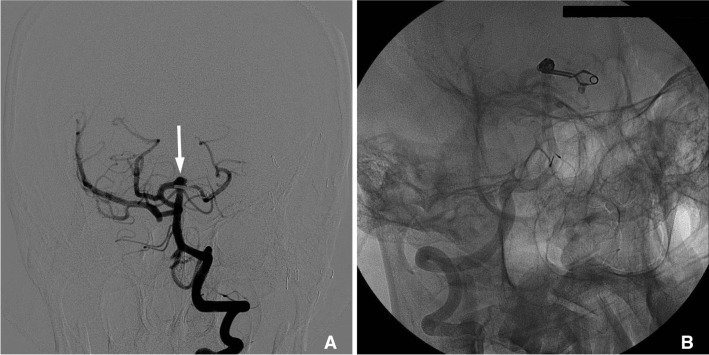
Fig. 2
Cerebral angiography reveals de novo aneurysm of left anterior choroidal artery (arrow) (A). One microcatheter (Echelon 10) was introduced into aneurysm (B). Cerebral angiography shows nearly total obliterated aneurysm and preserved anterior choroidal artery (C).
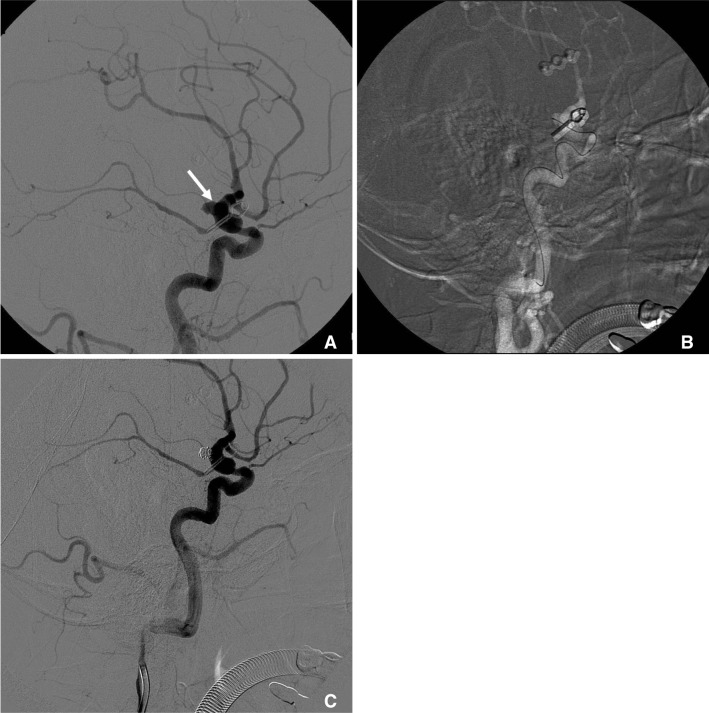
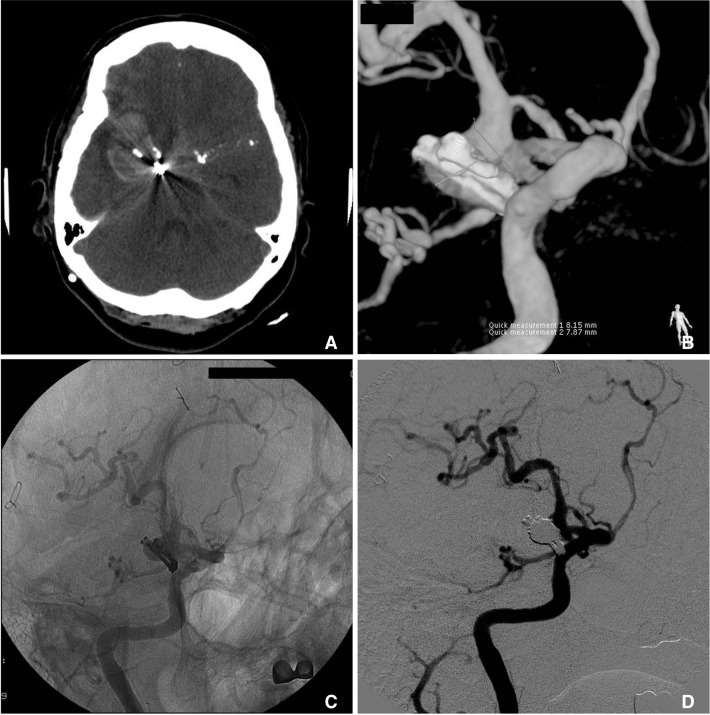
Fig. 3
Before coiling, aneurysm clip and thick subarachnoid hemorrhage are seen in brain CT scan (A). Cerebral angiography reveals regrowth of right P-com artery aneurysm (B, C). Cerebral angiography shows nearly total obliteration (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download