This article has been corrected. See "Author Correction: MRI Vessel Wall Imaging and Treatment of an Aneurysm at the Atlanto-Axial Segment of an Aberrant Vertebral Artery" in Volume 13 on page 145.
Abstract
We report a case of unique location of an aneurysm at the atlanto-axial extradural segment of a unilateral aberrant vertebral artery. The MRI vessel wall imaging findings and possible mechanism of aneurysm formation were discussed. A 5 mm extracranial vertebral artery aneurysm located at the interlaminar space between C1 and C2 was diagnosed in a woman presenting with occipital headache. The index vertebral artery ran an aberrant course at the V3 segment, where it entered the dura between C1 and C2 instead of the usual atlanto-occipital space. MR vessel wall imaging showed homogenous wall enhancement of the aneurysm sac. We surmise the anomalous course of the vertebral artery subjected the V3 segment to repeated shearing force secondary to the atlanto-axial rotational neck movement. This led to vessel wall trauma and inflammation, and subsequent aneurysm formation. The aneurysm was successfully treated with endovascular coiling with resolution of symptoms.
Extracranial vertebral artery (VA) aneurysm is rare and typically associates with penetrating neck trauma, neurofibromatosis, connective tissue diseases and syphilis.1 Its presentations are variable and underlying pathophysiology is not well described in the literature. Recently, utilizing the MR vessel wall imaging technology, we were able to correlate a unique case of external VA aneurysm with the underlying aberrant VA at the atlantoaxial junction. The potential mechanism of aneurysm formation, symptomatology, and treatment implications were discussed.
A 40-year-old woman with unremarkable health history presented with progressive occipital headache for a month. There was no history of head and neck trauma and there were no other symptoms. Physical examination revealed a tender area over the left posterior neck, but no signs of meningeal irritation or neurological deficit.
Catheter angiography showed an aberrant course of the V3 segment of left VA and a 5 mm unruptured extradural saccular aneurysm at the atlanto-axial level. The left VA enters the C6 transverse foramen and passes up along the course of usual anatomy from C6 to C2. However, it curves medially as it exits the C2 transverse foramen, and enters the dural sac after coursing through the narrow inter-laminar space between atlas and axis, where the aneurysm was located (Fig. 1). 3T MRI with proton-density and T1 Volume Isotropic Turbo spin echo Acquisition (PD and T1 VISTA, Philips Healthcare, The Netherlands) was used to visualize the aneurysm wall in a three-dimensional manner. The PD-VISTA and T1 post-gadolinium contrast VISTA showed homogenous circumferential enhancement in the aneurysm wall (Fig. 2).
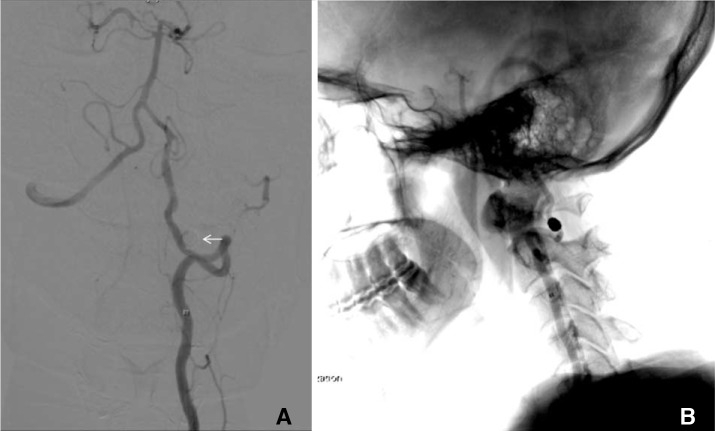
The patient underwent primary endovascular coiling for the aneurysm treatment via femoral artery access. The aneurysm was entered with Excelsior® SL-10 (Stryker, Kalamazoo, MI, USA) J-shaped microcatheter, and completely occluded with five Target® detachable coils (Stryker). There was no complication and the patient's headache resolved gradually. MR angiogram was repeated six months after the procedure and showed complete exclusion of the aberrant VA aneurysm with no reconstitution (Fig. 3).
Aberrant course of VA is a rare but important anatomical variation. This has been implicated in cervical myelopathy, stroke and cervical screw fixation complications.234 Depending on the segment of the aberrancy and its effect on the nearby structures, treatment hugely varies from patient to patient. The aberrant course of VA in our case is the result of embryological persistence of type II pro-atlantal artery and the complete regression of the usual distal VA segment. Its unique location at the narrow atlantoaxial inter-laminar space portends a potential risk of vessel wall injury with repeated neck rotation. In normal individual, the atlantoaxial joint allows rotation for up to 50 degrees, and this accounts for 50% of the cervical spine rotation range.5 To protect the artery from overstretching during neck movement, the usual VA loop between the transverse foramina of C1 and C2 is appropriately overlengthened.6 However, with aberrant course of the V3 loop, this protection is lost and movement across the joint has become a direct shearing stress to the vessel wall. Because of this repeated minor trauma, vessel wall weakening and inflammation ensued, and led to subsequent aneurysm formation.
This is evidenced by the patient's age of onset, symptomatology and the MR vessel wall imaging finding of aneurysm wall enhancement. In children and young adults, high arterial elasticity compensates the functional deformities of VA better than older individuals6, and this explains the delayed aneurysm formation at middle age and the subacute presentation of occipital neuralgia in our patient. On the other hand, current evidence with vessel wall imaging on aneurysm suggests that not all aneurysms will show vessel wall enhancement.7 Indeed, the degree of wall enhancement was found to be corresponding to the inflammatory activity in the vessel wall.78 Histopathology of both ruptured and unruptured intracranial aneurysms confirmed dense inflammatory cells infiltration at the aneurysm sac,910 and this correlates to the areas of enhancement on vessel wall imaging well.9 Therefore, the wall enhancement pattern in our patient likely represented the inflammation secondary to minor trauma and recent aneurysm formation at C1/2.
MRI vessel wall imaging, we utilized three dimensional (3D) VISTA with the following imaging parameters: TR/TE-2000/38 milliseconds, Turbo spin echo (TSE) factor-60 including 4 start-up echoes, SENSE factor-2, over-sampling factor-1.8, number of averages-1, acquired voxel volume 0.5×0.5×0.5 mm.3 The technique is a variant of TSE with variable flip-angle (FA) nonselective refocusing radiofrequency pulses and radial view ordering. The intrinsic black-pool effects of 3D VISTA were achieved by intra-voxel dephasing of moving blood spins, use of low FA refocusing pulses which causes the formation of stimulated echoes resulting in signal loss, and a smaller FA leads to greater flow suppression.11
Endovascular treatment was preferred in this patient as open repair of this aneurysm would require a farlateral approach and extensive suboccipital dissection, as well as hemi-laminectomy of C1 and C2 to obtain adequate vascular control proximal and distal to the aneurysm. Primary coiling was used instead and successfully obliterated the aneurysm and relieved patient's symptoms. The use of flow-diverter device had the theoretical advantage of reducing the aneurysm mass effect and compression of the occipital nerves more effectively. However, as the disease vessel segment was prone to repeated neck movement, which increased the risk of stent fracture or migration, stent devices were avoided.
References
1. Shang EK, Fairman RM, Foley PJ, Jackson BM. Endovascular treatment of a symptomatic extracranial vertebral artery aneurysm. J Vasc Surg. 2013; 58:1391–1393. PMID: 23561429.

2. Siclari F, Burger IM, Fasel JH, Gailloud P. Developmental anatomy of the distal vertebral artery in relationship to variants of the posterior and lateral spinal arterial systems. AJNR Am J Neuroradiol. 2007; 28:1185–1190. PMID: 17569985.

3. Tsang AC, Tsang FC, Lui WM. Bilateral aberrant C1/2 intradural vertebral arteries: a rare cause of cervical myelopathy. ANZ J Surg. 2017; 87:202–203. PMID: 25267558.

4. Chung JC, Jung SS, Park KS, Ha HG. Intraoperative vertebral artery angiography to guide c1-2 transarticular screw fixation in a patient with athethoid cerebral palsy. J Korean Neurosurg Soc. 2012; 51:177–181. PMID: 22639719.
5. Roche CJ, King SJ, Dangerfield PH, Carty HM. The atlanto-axial joint: physiological range of rotation on MRI and CT. Clin Radiol. 2002; 57:103–108. PMID: 11977941.

6. Penning L. Normal movement of the cervical spine. AJR Am J Roentgenol. 1978; 130:317–326. PMID: 414586.
7. Edjlali M, Gentric JC, Regent-Rodriguez C, Trystram D, Hassen WB, Lion S, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014; 45:3704–3706. PMID: 25325912.

8. Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol. 2016; 26:277–283. PMID: 25332151.

9. Hu P, Yang Q, Wang DD, Guan SC, Zhang HQ. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology. 2016; 58:979–985. PMID: 27438805.

10. Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, et al. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol. 2011; 7:547–559. PMID: 21931350.

11. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intra-cranial arterial wall imaging using three-dimensional high isotropic resolution black port MRI at 3 Tesla. J Magn Reson Imaging. 2011; 34:22–30. PMID: 21698704.
Fig. 1
Catheter angiogram with left vertebral artery injection. (A) Anteroposterior view showing a 5mm saccular aneurysm (arrow) at the extra-dural segment of the vertebral artery. (B) Lateral projection angiogram with fluoroscopy overlay showing the aneurysm (arrow) located at the atlantoaxial level.
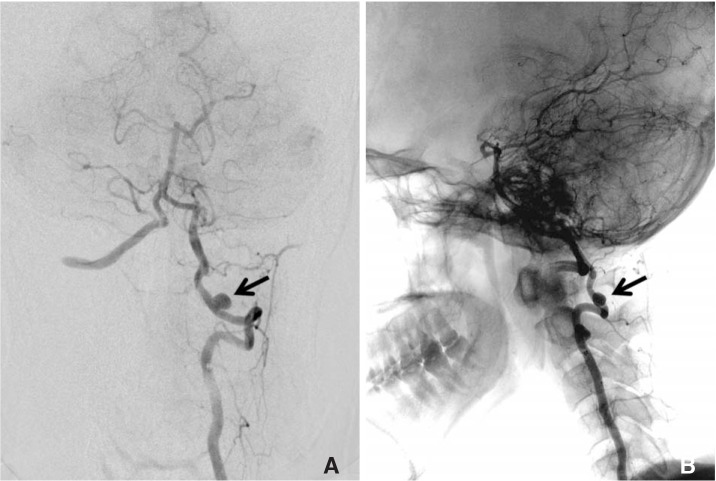
Fig. 2
Coronal MRI showing the aberrant left vertebral artery with the aneurysm located between the left atlas and axis (arrow). (A) PD VISTA and (B) T1-weighted with gadolinium contrast sequence showing circumferential aneurysm wall enhancement. Note the right vertebral artery followed the usual course above the atlas (arrowhead).
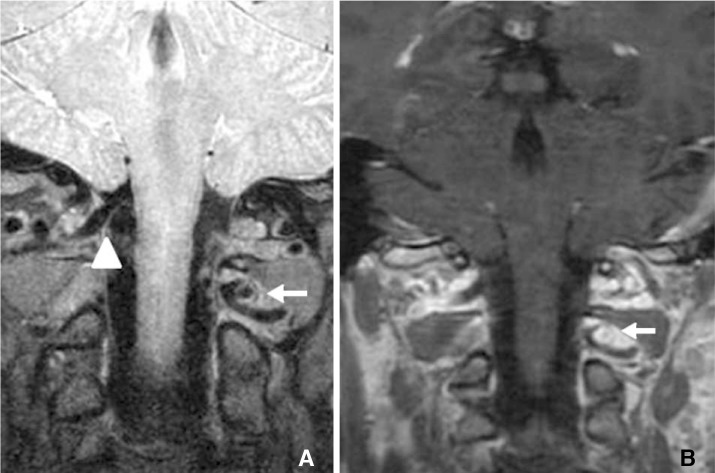
Fig. 3
(A) Control left vertebral artery subtraction angiogram showing its aberrant course, and complete exclusion of aneurysm from the circulation after coil embolization. (B) Lateral projection of the same left vertebral artery angiogram with fluoroscopy overlay. The aneurysm coil mass is located at the interlaminar space between C1 and C2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download