This article has been corrected. See "Author Correction: Treatment of Intracranial Aneurysms with the Pipeline Embolization Device Only: a Single Center Experience" in Volume 13 on page 144.
Abstract
Purpose
The aim of this study was to evaluate the technical feasibility and rate of mid-term occlusion in aneurysms treated solely with the Pipeline Embolization Device (PED) in a German tertiary care university hospital.
Materials and Methods
Forty-nine non-consecutive intracranial aneurysms underwent endovascular treatment using the PED exclusively between March 2011 and May 2017 at our institution. Primary endpoint was a favorable aneurysm occlusion defined as OKM C1-3 and D (O'Kelly Marotta Scale). Secondary endpoints were retreatment rate and delayed complications. Median follow-up was 200 days.
Results
The mean aneurysm size was 7.1 ± 5.3 mm. Forty-four aneurysms were located in the anterior circulation (90%). Ten aneurysms were ruptured (20%). Branching vessels from the sac were observed in 11 aneurysms (22%). Favorable obliteration immediately after PED placement was seen in 13/49 aneurysms (27%), of those nine aneurysms were completely occluded (18%). Angiographic and clinical follow-up was available for 45 cases (92%); 36/45 aneurysms (80%) were occluded completely and 40/45 aneurysms (89%) showed a favorable occlusion result. A branching vessel arising from the aneurysm sac was associated with incomplete occlusion (P < .05). All electively treated patients had good outcome (mRS 0). Three aneurysms (6%) required additional treatment due to aneurysm recurrence.
Endovascular therapy of intracranial aneurysms is a standard practice across neurovascular centers, most commonly by means of deployment of detachable coils into the aneurysm via a dedicated microcatheter as first described by Guglielmi.1 In aneurysms with wide neck or low dome-to-neck ratio (<2), adjunctive techniques such as balloon remodeling or stent placement are proven additional options.2 Recently, endoluminal parent vessel reconstruction via a flow diverter has been added to the range of treatment options and is routinely used in broad-based and fusiform aneurysms.3 The Pipeline Embolization Device (PED, Covidien, Irvine, CA, USA) as one such device received CE mark in 2008 and US Food and Drug Administra-tion (FDA) approval in 2011 for the treatment of large and giant wide-necked (≥ 4 mm or no discernible neck) aneurysms of the internal carotid artery (ICA) between petrous and superior hypophyseal segments. The device has proven to be eff icient in maintaining stable aneurysm occlusion with a favorable safety profile.4,5,6,7,8,9,10,11 However, increasing operator experience and promising long-term outcome data have contributed to a further array of uses such as posterior circulation aneurysms or ruptured aneurysms.12 Thus, there are current discussions about the use of flow diverters, for example the consequence of subsequent dual antiplatelet therapy with the potential risk for hemorrhagic complications in patients with ruptured aneurysms related to aneurysmal rebleeding and associated surgical procedures such as ventriculostomy, hemicraniectomy or perforator occlusion in the brainstem due to low porosity and potential trans-branch-occlusion of flow diverters.1314
We therefore systematically analyzed the results of those intracranial aneurysm treatments with exclusive use of the PED without adjunctive coiling in our tertiary care center.
Eighty-nine patients harboring 93 intracranial aneurysms were treated with 169 PEDs at our institution between March 2011 and May 2017. Forty-nine aneurysms in 48 patients non-consecutive underwent endovascular treatment using the PED exclusively, meaning no adjunctive techniques were used. Forty-one aneurysms were addressed with a PED as first-line therapy. However, eight patients with recurrence or primary incomplete occlusion during conventional coiling or surgical clipping were included in the analysis. Non-ruptured and ruptured aneurysms located in the anterior and posterior circulation were included. Patients primarily treated with the PED in conjunction with coil embolization as well as bifurcation aneurysms were excluded from the study. Portions of the data of included patients are reported in an article that focuses on 1) the comparison of safety and efficacy of nonstaged use of multiple PEDs versus single PED in cerebral aneurysm treatment and 2) the feasibility of flow diverter therapy in dissecting aneurysms in the posterior circulation.1516 All indications were based on an interdisciplinary decision making between neurosurgeons and interventional neuroradiologists. In patients with subarachnoid hemorrhage (SAH; Fisher >2), Hunt and Hess grade ≥3 or evidence of hydrocephalus, an external ventricular drainage was performed prior to endovascular treatment. Data was derived from a prospectively kept database, anonymized and analyzed retrospectively. According to the guidelines of the respective local ethics committees, no approval was necessary for this anonymous retrospective study, which was conducted in accordance to the Declaration of Helsinki. The primary endpoint was a favorable aneurysm closure defined as O'Kelly Marotta (OKM) scale C1−3+D.17 The secondary endpoint was retreatment and delayed complications. An incomplete occlusion was defined as OKM A1-B3 on patient's final follow-up angiogram. All procedural complications were reported regardless of their clinical significance.
Patients undergoing elective PED therapy received double antiplatelet medication (75 mg/d clopidogrel, 100 mg/d aspirin (ASA)) starting 5 days before the intervention and maintained for 3 months post treatment, followed by continuous ASA single antiplatelet therapy for life. Platelet function tests were routinely performed using ASA and P2Y12 assays (VerifyNow, Accumetrics, San Diego, CA, USA). A platelet inhibition level between 30–90% for clopidogrel and 350–550 response units for ASA was defined as acceptable. Poor responders to clopidogrel were either counteracted by dose escalation (e.g., clopidogrel 150 mg/d) or switched to prasugrel (40 mg loading dose, 5 mg/d). In patients with SAH infusion of tirofiban (Aggrastat®, Merck, West Point, PA, USA) was administered intraoperatively according to the manufacturer's guidelines, followed by ASA and clopidogrel 16–24 hours later after a control noncontrast computed tomography (CT). A bolus of heparin (5,000 IU) was administered after groin puncture, followed by aliquots of 1,000 IU/h. Activated clotting time was maintained at 2–3 times the patient's baseline intraoperatively. Heparin was discontinued at the end of the procedure.
Procedures were performed under general anesthesia. Femoral access was obtained with a short 8F femoral sheath. Three-dimensional rotational angiography was executed in all patients for the determination of the ideal working projection. As described previously, all PEDs were deployed through a dedicated microcatheter (Marksman, Medtronic, Minneapolis, MN, USA) using a biaxial guide-catheter system applying a push-pull technique and aiming a maximal mesh density across the aneurysm neck with avoiding oversizing of the implant.18 The number of flow diverter deployed was left to the operator's discretion and based on the angiographic evaluation of intra-aneurysmal contrast agent stasis. If possible, it was avoided to deploy more than one flow diverter stent, especially in the basilar artery, in order to minimize the risk of a perforator infarction. Overlapping multiple PEDs implantation was typically conducted in fusiform aneurysms and in cases of unchanged blood flow within the aneurysm after single PED deployment.
Patients with primarily ruptured aneurysms underwent CT within 24 hours to exclude rehemorrhage and periprocedural ischemic complications. Follow-up was performed by digital subtraction angiography at 3–6 months and two years after treatment. The clinical outcome (modified Rankins score, mRs) was established by a consultant neurosurgeon at the time of the follow-up angiography.
Statistical analysis was conducted using JMP Software (V12.0, SAS Institute, Cary, NC, USA). Statistical differences were calculated using the Wilcoxon Test. Statistical significance was set to P <.05. Baseline statistics are presented as the median or mean and range for continuous variables, and as frequency and percent for categorical variables.
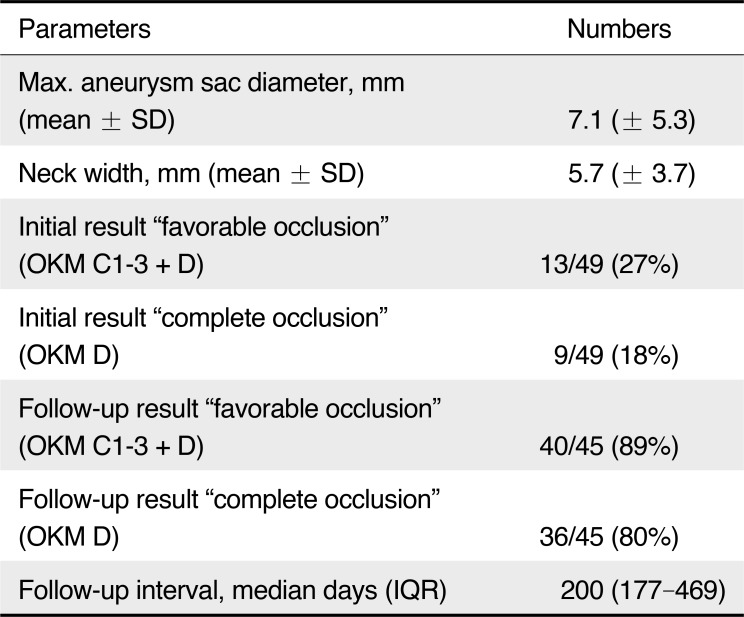
Forty-eight patients with 49 intracranial wide-neck aneurysms, including 41 women and seven men underwent PED placement (Table 1). The patient age ranged from 26 to 84 years (mean 53.4 ± 11.8). Mean aneurysm size was 7.1 ± 5.3 mm. Thirty-seven aneurysms were <10 mm (76%) and 12 aneurysms ≥ 10 mm (24%) including one giant aneurysm >25 mm. Neck width was 5.7 ± 3.7 mm on average with a dome-to-neck ratio of 1.1 ± 0.4. Morphologically, 39 aneurysms were saccular (80%), five were fusiform or dissecting (10%) and f ive were blister aneurysms (10%). Forty-four aneurysms were located in the anterior circulation (90%) and 10 aneurysms were ruptured (20%). In 11 aneurysms (22%) a branch vessel arose from the aneurysm sac.
A total of 80 PEDs were implanted, from those 28 aneurysms (57%) were treated with a single PED, while 21 aneurysms (43%) were treated with two or more PEDs. Average number of PED used was 1.6 ± 0.8. No adjunctive coil placement was performed. Device placement was successful in 98% (Fig. 1); in a patient with a ruptured P2/3 aneurysm a loss of the capture coil followed by a rescue maneuver resulted in a vessel perforation with subsequent coiling and sacrifice of the parent artery. The patient underwent hemicraniectomy due to initial SAH and suffered from severe vasospasms with symptomatic infarction despite intraarterial therapy with nimodipine. Seven patients (14%) required balloon angioplasty because of inadequate PED expansion. Favorable obliteration (OKM C1−3+D) immediately after PED placement was observed in 13 aneurysms (27%), of those nine aneurysms were completely occluded (18%, Table 2). In one patient with a posterior communicating aneurysm an in-stent-occlusion in a two-layer PED construct on the third day after placement was observed. The patient remained asymptomatic due to good collateralization. Mortality rate was 4%; two patients with ruptured aneurysms in the posterior circulation died perioperatively due to severe SAH. However, no device-related mortality was observed. No further procedural complications such as aneurysm rupture, side branch occlusion or thrombembolism occurred.
The median follow-up was 200 days (interquartile range 177 to 469 days). Angiographic and clinical follow-up was available for 45 aneurysms (92%). Thereof, 36 aneurysms (80%) were completely occluded (OKM D) and 40 aneurysms (89%) showed a favorable occlusion result (OKM C1−3+D); of the five non-occluded aneurysms (11%), two patients each with a paraophthalmic aneurysm showed a branch vessel arising from the aneurysm sac (Fig. 2). This was statistically significant for causing incomplete occlusion (P <.05). No delayed aneurysm rupture occurred. Six out of eight previously treated aneurysms were occluded completely with PED as a second-line therapy (75%). All electively treated patients showed an excellent outcome (mRS 0). In acute SAH cases seven patients (70%) had a favorable outcome (mRS ≤2) during ollow-up.
Overall, retreatment was necessary in three patients (6%) due to aneurysm recurrence in the perioperative period. These cases were successfully treated by implantation of additional PEDs.
The implantation of flow diverters is a well-established treatment option in patients with intracranial aneurysms by directing flow along the lumen of the parent vessel inducing intraaneurysmal stasis with subsequent thrombosis while maintaining perfusion to side branches.4567891011 Furthermore, flow diverter devices serve as a scaffold permitting neo-intimal overgrowth across the aneurysm neck which lead to remodeling of the deficient segment with exclusion of the aneurysm from the circulation.19 The PED is an endoluminal and self-expanding braided device, comprised of platinum (25%) and cobalt-nickel alloy (75%) and acts like a low-porosity stent with a 35% metallic surface area coverage when fully deployed.20 The technical feasibility and resulting aneurysm occlusion rates have been demonstrated in large studies previously.621 In our single center study including patients treated without adjunctive devices the PED proved to be a safe and effective option with a high aneurysm occlusion rate in absence of severe procedure-associated complications.
A favorable aneurysm occlusion rate (AOR) of 88% in the follow-up with a device-related mortality of 0% in our study is in accordance with recent reports with regard to treatment efficacy.91122 The AOR increased over time, which is common after PED placement as a result of gradual closure. In a meta-analysis of 13 studies including 1043 intracranial aneurysms treated with PED the AOR after six months was 79.7%, however, studies with longer follow-up periods showed a progressive increase in AOR up to 85% with time.222324 Different predictive factors for delayed or incomplete aneurysmal occlusion are part of the current debate. In our cohort, the presence of a branch vessel arising from the aneurysm sac was statistically associated with incomplete closure of the aneurysm after flow diverter implantation in the patient's follow-up angiogram. This observation accompanies with the results of Chiu et al., who found a delayed mean time of occlusion from nine months due to vessel incorporation.25 One possible explanation for this finding might be a reduction of the efficacy of clot formation due to a preserved flow in the aneurysm sac.
Two patients in our study (angiographic result OKM A2 and B3, respectively) opted for conservative MRI-based surveillance. Delayed aneurysm rupture is a serious possible complication of flow diverter therapy and was described in approximately 1% of cases in the literature, especially in large or giant aneurysms;26 however, it was not observed in our cohort. This might be explained due to the limited number of patients. The pathophysiological mechanism of delayed aneurysm rupture may include hemodynamic changes in aneurysmal flow patterns or autolysis caused by the release of proteolytic enzymes within the thrombus, resulting in subsequent aneurysm wall weakening.5 An important clinical consideration is that in case of a delayed rupture, therapeutic strategies are limited because a two-staged coiling after PED placement is precluded due to its small pore size. Seventy-six percent of aneurysms in our cohort were <10 mm suggesting that delayed device-related rupture is a rare phenomenon in smaller aneurysms.25
The rate of spontaneous intraparenchymal hemorrhage (sIPH) in our study was 2% and is in line to the rates reported in literature.3 The underlying pathophysiological mechanism for sIPH may include hemorrhagic transformation of ischemic stroke, hemodynamic alteration due to flow diverter placement, potential association with intraprocedural foreign body emboli and dual antiplatelet therapy.5 In this context special attention is given to the treatment of ruptured aneurysms with PED. However, published literature is limited and US FDA approval for its use in ruptured aneurysms is still pending. The main issue is that patients with SAH frequently require additional intracranial procedures such as ventriculostomy or hemicraniectomy with a potential risk for hemorrhage under ongoing antiplatelet therapy.27 Chalouhi et al. reported the successful treatment of ruptured aneurysms with PED yielding a good outcome (mRS ≤2) in 95% with a fatal aneurysm rupture in only a single patient.28 Another multicenter study demonstrated a periprocedural complication rate of 19% including three deaths and mRS ≤2 in 77% of patients.27 Our results with seven patients (70%) having a good clinical outcome after rupture are marginally lower due to the limited sample size. This emphasizes that seriously affected patients suffer from bleeding alone prior to treatment. Given the relatively higher rate of complications in literature, many centers tend to coil the ruptured aneurysm followed by staged flow diversion, however, in our select cases no adjunctive techniques were used.29
In-stent stenosis was not observed in any patient during follow-up, however, one patient suffered from an asymptomatic PED occlusion on the 3rd day after implantation despite a suff icient anticoagulation therapy. The reason remains unclear; the enlarged amount of thrombogenic metal in a two-layer PED construct could be a potential explanation. In most cases PED occlusion is observed later, primarily when clopidogrel is ceased.25
Ischemic strokes due to side branch occlusion are a potential risk after PED placement although computational analysis has shown a flow reduction of only less than 10% when the perforating vessel ostium is covered up to 90%.30 An overall side branch occlusion rate of 2.3% is reported in literature.23 Kallmes et al. found an ischemic event rate of 4.7% with higher rates in posterior circulation aneurysms.21 For this reason some investigators suggest preventing the use of multiple flow diverters, especially in the vertebrobasilar vasculature.926 In our study no perforator infarction was observed, although four patients with dissecting and blister aneurysms in the posterior circulation were treated with more than one PED.
Seventy-five percent of our patients treated with PED as a second-line therapy showed a complete aneurysm occlusion. This is superior to the results published in literature ranging from 56 to 65%.3132 However, this result should be considered with caution since all types of pre-treatment were summarized in our small cohort. No technical difficulties occurred, but in 38% of cases apposition of the PED to the vessel wall was improved with balloon angioplasty.
A major limitation of this study is the retrospective design with the attendant selection bias, a small sample size, and heterogeneity of any such population presented here and an absence of a control group. This study reports the experience of a single high-volume center with specific techniques and protocols. Thus, results may not be generalizable. Despite these limitations we were able to confirm that the presence of a branch vessel arising from the aneurysm sac is a predictive factor for an incomplete occlusion during follow-up.
In our experience, primary treatment of intracranial aneurysms with PED without adjunctive coiling was associated with low complications rates and high aneurysm occlusion rates at mid-term follow-up. The presence of an incorporated vessel was significantly associated with incomplete aneurysm occlusion, but most aneurysms still do occlude over time. In-stent thrombosis is a rare phenomenon even under ongoing sufficient antiplatelet therapy and may be asymptomatic. Randomized trials with long-term follow-up are necessary to extend the therapeutic options, especially in the posterior circulation and in ruptured aneurysms.
References
1. Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991; 75:1–7. PMID: 2045891.
2. Naggara ON, Lecler A, Oppenheim C, Meder JF, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: a systematic review of the literature on safety with emphasis on subgroup analyses. Radiology. 2012; 263:828–835. PMID: 22623696.

3. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–447. PMID: 23321438.

4. Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Randazzo C, et al. The Pipeline Embolization Device: learning curve and predictors of complications and aneurysm obliteration. Neurosurgery. 2013; 73:113–120. PMID: 23615106.
5. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke. 2013; 44:2046–2054. PMID: 23798560.
6. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013; 267:858–868. PMID: 23418004.

7. Chalouhi N, Starke RM, Yang S, Bovenzi CD, Tjoumakaris S, Hasan D, et al. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke. 2014; 45:54–58. PMID: 24253543.

8. Kan P, Siddiqui AH, Veznedaroglu E, Liebman KM, Binning MJ, Dumont TM, et al. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: a U.S. multicenter experience. Neurosurgery. 2012; 71:1080–1087. PMID: 22948199.
9. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 32:34–40. PMID: 21148256.

10. McAuliffe W, Wycoco V, Rice H, Phatouros C, Singh TJ, Wenderoth J. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2012; 33:164–170. PMID: 21979492.

11. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012; 33:1436–1446. PMID: 22821921.

12. Murthy SB, Shah J, Mangat HS. Treatment of Intracranial Aneurysms With Pipeline Embolization Device: Newer Applications and Technical Advances. Curr Treat Options Neurol. 2016; 18:16. PMID: 26923606.

13. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010; 31:1139–1147. PMID: 20150304.

14. Amenta PS, Dalyai RT, Kung D, Toporowski A, Chandela S, Hasan D, et al. Stent-assisted coiling of wide-necked aneurysms in the setting of acute subarachnoid hemorrhage: experience in 65 patients. Neurosurgery. 2012; 70:1415–1429. PMID: 22186840.
15. Kabbasch C, Mpotsaris A, Behme D, Dorn F, Stavrinou P. Pipeline Embolization Device for Treatment of Intracranial Aneurysms-The More, the Better? A Single-center Retrospective Observational Study. J Vasc Interv Neurol. 2016; 9:14–20. PMID: 27829966.
16. Maus V, Mpotsaris A, Dorn F, Mohlenbruch M, Borggrefe J, Stavrinou P, et al. The Use of Flow-Diverter in Ruptured, Dissecting Intracranial Aneurysms of the Posterior Circulation. World Neurosurg. 2017; 12. 23. S1878-8750(17)32211-8. DOI: 10.1016/J.wneu.2017.12.095. [Epub ahead of printing].
17. O'Kelly C J, Krings T, Fiorella D. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010; 16:133–137. PMID: 20642887.
18. Shapiro M, Raz E, Becske T, Nelson PK. Building multidevice pipeline constructs of favorable metal coverage: a practical guide. AJNR Am J Neuroradiol. 2014; 35:1556–1561. PMID: 24676003.

19. Lopes D. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005; 56:E416. PMID: 15670395.

20. Krishna C, Sonig A, Natarajan SK, Siddiqui AH. The expanding realm of endovascular neurosurgery: flow diversion for cerebral aneurysm management. Methodist Debakey Cardiovasc J. 2014; 10:214–219. PMID: 25624975.
21. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafe A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015; 36:108–115. PMID: 25355814.

22. O'Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2013; 34:381–387. PMID: 22859284.
23. Murthy SB, Shah S, Venkatasubba Rao CP, Bershad EM, Suarez JI. Treatment of unruptured intracranial aneurysms with the pipeline embolization device. J Clin Neurosci. 2014; 21:6–11. PMID: 24055205.

24. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 265:893–901. PMID: 22996749.

25. Chiu AH, Cheung AK, Wenderoth JD, De Villiers L, Rice H, Phatouros CC, et al. Long-Term Follow-Up Results following Elective Treatment of Unruptured Intracranial Aneurysms with the Pipeline Embolization Device. AJNR Am J Neuroradiol. 2015; 36:1728–1734. PMID: 25999412.

26. Chalouhi N, Tjoumakaris S, Phillips JL, Starke RM, Hasan D, Wu C, et al. A single pipeline embolization device is sufficient for treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2014; 35:1562–1566. PMID: 24788125.

27. Lin N, Brouillard AM, Keigher KM, Lopes DK, Binning MJ, Liebman KM, et al. Utilization of Pipeline embolization device for treatment of ruptured intracranial aneurysms: US multicenter experience. J Neurointerv Surg. 2015; 7:808–815. PMID: 25230839.

28. Chalouhi N, Zanaty M, Whiting A, Tjoumakaris S, Hasan D, Ajiboye N, et al. Treatment of ruptured intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2015; 76:165–172. PMID: 25549187.

29. Brinjikji W, Piano M, Fang S, Pero G, Kallmes DF, Quilici L, et al. Treatment of ruptured complex and large/giant ruptured cerebral aneurysms by acute coiling followed by staged flow diversion. J Neurosurg. 2016; 125:120–127. PMID: 26654182.

30. Mut F, Lohner R, Chien A, Tateshima S, Viñnuela F, Putman C, et al. Computational Hemodynamics Framework for the Analysis of Cerebral Aneurysms. Int J Numer Method Biomed Eng. 2011; 27:822–839. PMID: 21643491.

31. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bazner H, Henkes H. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012; 54:369–382. PMID: 21881914.

32. Daou B, Starke RM, Chalouhi N, Tjoumakaris S, Hasan D, Khoury J, et al. Pipeline Embolization Device in the Treatment of Recurrent Previously Stented Cerebral Aneurysms. AJNR Am J Neuroradiol. 2016; 37:849–855. PMID: 26611991.

Fig. 1
Angiogram of a 56-year-old patient (no. 44) shows a saccular supraophthalmic LICA aneurysm with a maximal sac diameter of 4 mm (A, black arrow). Immediately after PED placement (B, C) the intra-aneurysmal filling grade is still >95% (OKM A2, C, arrow). At follow-up after six months the aneurysm is completely occluded (OKM D, D).

Fig. 2
Recurrence of a paraophthalmic LICA aneurysm in a 53-year-old patient (no. 27) after previous coiling (black arrow) and complete occluded RMCA aneurysm after surgery (A). After PED placement (B) the final angiogram showed a partially occluded aneurysm (OKM B3, image not shown). Approximately eight months after placement no significant change of filling and stasis grades was observed (C).
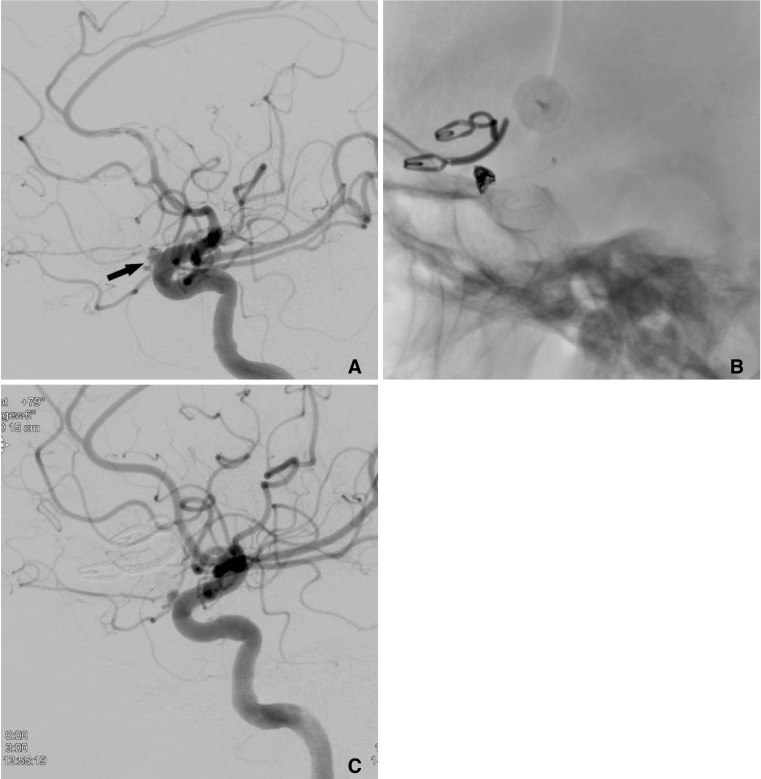
Table 1
Baseline, Clinical, and Angiographic Characteristics
OKM, O'Kelly Marotta Scale; PED, Pipeline embolizaion device; d, days; f, female; m, male; r, right; l, left; ICA, internal carotid artery; PCA, posterior cerebral artery; VA, vertebral artery; Pcom, posterior communicating artery; Acom, anterior communicating artery; MCA, middle cerebral artery; PICA, posterior cerebellar artery
*Retreatment necessary due to aneurysm recurrence during follow-up
Table 2
Overview of Angiographic Outcome after PED Implantation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download