Abstract
Formation of de novo aneurysm from a junctional dilatation at the origin site of the posterior communicating artery (PcomA) has been rarely reported. In this case report, three females in sixth decades of age developed a de novo aneurysm from the junctional dilatation of the PComA with a tiny bleb-like lesion over 5 years after initial presentation.
Diffuse dilatation at the origin site of the posterior communicating artery (PcomA) is known as a "junctional dilatation" or an "infundibulum". Usually, the PcomA is originated from the apex of the junctional dilatation and it has a triangular shape [12]. The incidence has been reported to be up to 17% of catheter angiography [3]. There was no proven histological abnormalities in an autopsy study of junctional dilatation at the PcomA [4]. Epstein et al. reported that seven junctional dilatations at the origin of the PcomA from routine autopsy revealed that adventitia, media, internal elastic membrane, and intima were intact. They concluded that the junctional dilatation at the PcomA was a normal variation and not a site having predilection to bleeding or future true aneurysmal dilatation.
Although the junctional dilatation at the PcomA is mostly considered to be a benign variant, several reports have documented the evolution of such a dilatation to a saccular de novo aneurysm with subsequent bleeding especially at the PcomA [56]. Therefore, the management and follow-up of an incidentally diagnosed junctional dilatation at the PcomA remains uncertain whether there is no possibility to develop aneurysm at all or not. We presented three patients who showed transformation from junctional dilatations at the PcomA to a saccular aneurysm and report characteristic finding on 3D angiography which has not been described previously.
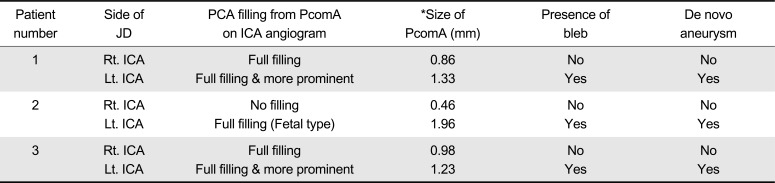
Summary of three patients was shown in Table 1. Detailed angiographic findings were compared in Table 2.
A 53-year-old healthy female patient developed an acute onset of severe occipital headache. Computed tomography (CT) scans showed a subarachnoid hemorrhage (SAH) mainly around the brainstem. Cerebral angiography revealed a saccular aneurysm with a maximum diameter of 4 mm in the right superior cerebellar artery origin and junctional dilatation at both PcomA origins. 3D rotational angiography showed that bilobulated bleb in the left junctional dilatation (Fig. 1A). The right SCA aneurysm was then treated by clipping surgery. One-year follow-up CT angiogrphy showed that complete occlusion of right SCA aneurysm and no change of both side junctional dilatations at the PcomA origin. Five-year follow-up angiography revealed a de novo internal carotid artery (ICA) aneurysm developing from a junctional dilatation at the origin of the left PcomA (Fig. 1B). Considering the direction, shape and position of the bleb, demonstration of a bleb by 3-D rotational angiography at the dominant side of bilateral junctional dilatation of the P-comA seems to be an important risk factor for evolution of a newly developed aneurysm. The patient underwent another clipping surgery for this de novo aneurysm.
A 57-year-old healthy female presented with an acute onset of altered mentality. CT showed a SAH mainly noted around the left Sylvian f issure and cerebral angiography revealed a bilobulated aneurysm with a maximum diameter of 4 mm in the left MCA bifurcation and a round saccular aneurysm with a maximum diameter of 5 mm in the left anterior choroidal artery origin. In addition, there was a triangular junctional dilatation at the origin of the PcomA in both sides. The left sided PcomA origin with junctional dilatation possessed a tiny bleb (Fig. 2A). Both aneurysms at the left MCA and the left anterior choroidal artery were clipped on single stage. One-year follow-up CT angiography showed complete occlusions of the clipped aneurysms at the left MCA and the left anterior choroidal artery, and no change of junctional dilatations at both sides of the PcomA origin. Five-year follow-up 3D rotational angiography revealed a de novo aneurysm formation at a previous junctional dilatation at the origin of the left PcomA (Fig. 2B).
A 55-year-old healthy female patient presented with an acute onset of seizure like movement. CT angiography showed a SAH with the maximum around the left Sylvian fissure and revealed a saccular aneurysm with a maximum diameter of 8 mm in left MCA bifurcation origin. The left MCA aneurysm was treated by clipping. CT angiography also revealed a triangular junctional dilatation at the origin of both PcomA. 3D rotational angiography showed that the left sided junctional dilatation possessed a tiny bleb (Fig. 3A). One-year follow-up CT angiography showed complete occlusions of the left MCA aneurysm and no change of junctional dilatations at both sides of the PcomA origin. Five-year follow-up 3D rotational angiography revealed a de novo aneurysm formation developing from junctional dilatation at the origin of the left PcomA (Fig. 3B). The aneurysm was clipped.
Our study showed development of de novo aneurysm from junctional dilatation of the PcomA origin in three patients who had been followed for about 5 years after initial presentation. The initial 3D rotational angiography at the time of diagnosis showed a tiny bleb at the junctional dilatation, which subsequently developed into a de novo saccular aneurysm during about 5-year duration. To the best of our knowledge, this observation of a tiny bleb at the junctional dilatation detected by 3D rotational angiography has not been described in the literature. Therefore, 3D rotational angiography is recommended to detect any bleb-like irregularities especially at the dominant side of bilateral junctional dilatations of the PcomA.
The junctional dilatations of cerebral arteries are most frequently observed at the origin of the PcomA as shown in our cases. It can be found in the other locations such as the anterior choroidal artery, the ophthalmic artery, the middle cerebral artery and the superior cerebellar artery. For the criteria of junctional dilatation which was proposed by Taveras and Wood in 1964 [2], it was defined as follows ; 1) diameter less than 3 mm and 2) the PcomA emerges from the apex of the enlargement.
Review of previous literature revealed six cases with a development of aneurysm from pre-aneurysmal junctional dilatations of the PcomA [78910111213]. All six patients were female between 20 and 40 years of age. Five of them had a history of aneurysm involving other cerebral arteries. The junctional dilatations required 6 months to 9 years to develop into aneurysms. Some risk factors suggested for the evolution of de novo aneurysm from junctional dilatation were history of angiography-negative SAH, familial occurrence of intracranial aneurysms and known disease with an increased propensity to aneurysm formation (e.g., adult polycystic kidney degeneration, Ehlers-Danlos disease, aortic coarctation) [614]. All 3 de novo aneurysms of our case report were found to be unruptured aneurysms on routine follow-up study as the most of previously reported cases were. However, some authors reported a rupture of de novo aneurysms developing from the junctional dilatation [151617]. We do not know the exact time interval of the aneurysm development from junctional dilatation. The expected time period would be between one to five years in our cases.
In conclusion, our case showed that the junctional dilatation of PcomA can develop into a de novo aneurysm on long term follow-up. Notably, a tiny bleb noted on 3D rotational angiography can predispose to a subsequent de novo aneurysm at the junctional dilatation especially on the dominant side of PcomA.
References
1. Hassler O, Saltzman GF. Histologic changes in infundibular widening of the posterior communicating artery. A preliminary report. Acta Pathol Microbiol Scand. 1959; 46:305–312. PMID: 13852000.
2. Taveras JM, Wood EH. Diagnostic Neuroradiology. Baltimore: Williams and Wilkins;1964. p. 498.
3. Endo S, Furuichi S, Takaba M, Hirashima Y, Nishijima M, Takaku A. Clinical study of enlarged infundibular dilation of the origin of the posterior communicating artery. J Neurosurg. 1995; 83:421–425. PMID: 7666216.

4. Epstein F, Ransohoff J, Budzilovich GN. The clinical significance of junctional dilatation of the posterior communicating artery. J Neurosurg. 1970; 33:529–531. PMID: 5479491.

5. Itakura T, Ozaki F, Nakai E, Fujii T, Hayashi S, Komai N. Bilateral aneurysm formation developing from junctional dilatation (infundibulum) of the posterior communicating arteries. Case report. J Neurosurg. 1983; 58:117–119. PMID: 6847897.
6. Fischer S, Hopf N, Henkes H. Evolution from an infundibulum of the posterior communicating artery to a saccular aneurysm. Clin Neuroradiol. 2011; 21:87–90.

7. Drake CG. On the surgical treatment of ruptured intracranial aneurysms. Clin Neurosurg. 1965; 13:122–155. PMID: 5870662.
8. Stuntz JT, Ojemann GA, Alvord EC Jr. Radiographic and histologic demonstration of an aneurysm developing on the infundibulum of the posterior communicating artery. Case report. J Neurosurg. 1970; 33:591–595. PMID: 5479497.
9. Trasi S, Vincent LM, Zingesser LH. Development of aneurysm from infundibulum of posterior communicating artery with documentation of prior hemorrhage. AJNR Am J Neuroradiol. 1981; 2:368–370. PMID: 6787905.
10. Waga S, Morikawa A. Aneurysm developing on the infundibular widening of the posterior communicating artery. Surg Neurol. 1979; 11:125–127. PMID: 424981.
11. Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. Am J Roentgenol Radium Ther Nucl Med. 1967; 101:68–87.

12. Yoshimoto T, Suzuki J. Surgical treatment of an aneurysm on the funnel-shaped bulge of the posterior communicating artery. Case report. J Neurosurg. 1974; 41:377–379. PMID: 4415999.
13. Young B, Meacham WF, Allen JH. Documented enlargement and rupture of a small arterial sacculation. Case report. J Neurosurg. 1971; 34:814–817. PMID: 5561026.
14. Coupe NJ, Athwal RK, Marshman LA, Brydon HL. Subarachnoid hemorrhage emanating from a ruptured infundibulum: case report and literature review. Surg Neurol. 2007; 67:204–206. PMID: 17254894.
15. Marshman LA, Ward PJ, Walter PH, Dossetor RS. The progression of an infundibulum to aneurysm formation and rupture: case report and literature review. Neurosurgery. 1998; 43:1445–1448. discussion 1448-1449PMID: 9848859.

16. Radulovic D, Nestorovic B, Rakic M, Janosevic V. Enlargement to a saccular aneurysm and subsequent rupture of infundibular widening of posterior communicating artery. Neurochirurgie. 2006; 52:525–528. PMID: 17203900.

17. Takahashi C, Fukuda O, Hori E, Kameda H, Endo S. A case of infundibular dilatation developed into an aneurysm and rupturing after the rupture of an aneurysm 10 years ago. No Shinkei Geka. 2006; 34:613–617. PMID: 16768138.
Fig. 1
A 53-year-old female (Case 1). (A) Initial 3-D rotational angiogram shows a bilobulated bleb (arrowhead) at junctional dilatation of the posterior communicating artery (PcomA). (B) Follow-up 3-D rotational angiogram obtained 5 years later shows de novo aneurysm formation at the site of the previous bleb (arrowhead).
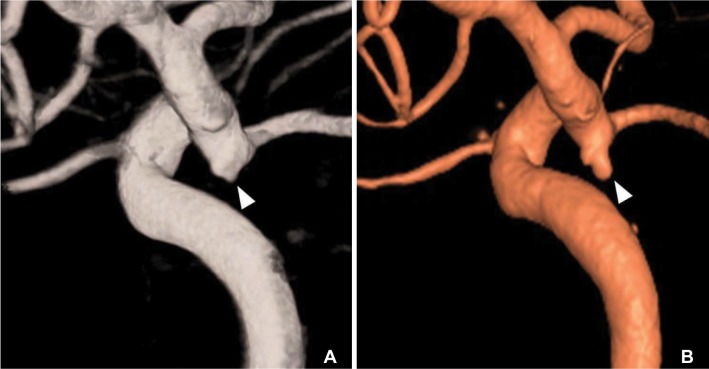
Fig. 2
A 57-year-old female (Case 2). (A) Initial angiogram showed a tiny bleb (arrowhead) at triangular junctional dilatation of the PcomA. (B) Follow-up angiogram obtained five years later revealed a de novo aneurysm formation from a junctional dilatation at the PcomA. It located just below the clipping site of the anterior choroidal artery aneurysm.
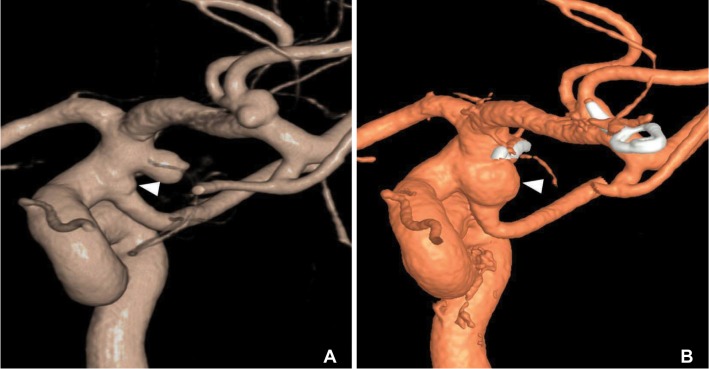
Fig. 3
A 55-year-old female (Case 3). (A) Initial angiogram showed a bleb (arrowhead) at junctional dilatation of the PcomA. (B) Follow-up angiogram obtained five years later revealed a de novo aneurysm (arrowhead) at the site of previous bleb.
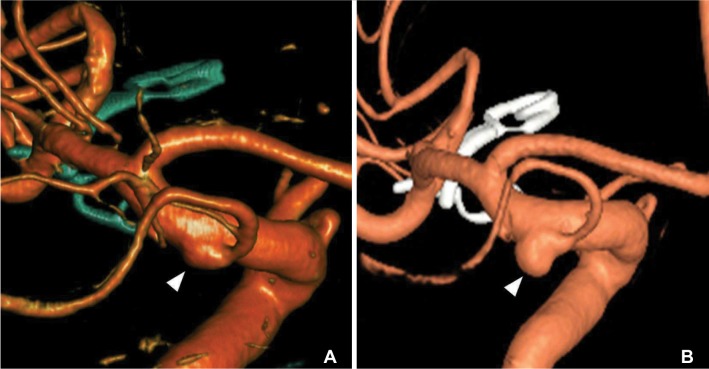
Table 1
Summary of Three Patients with De Novo Aneurysm Developing from Junctional Dilatation
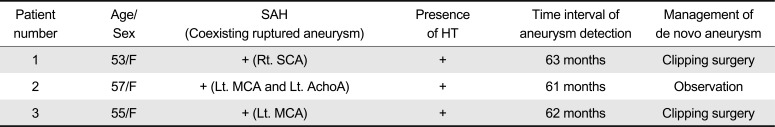
Table 2
The Comparison Between Bilateral Junctional Dilatations in Each Individual Case




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download