Transient cortical blindness (TCB) is a rare but well-known complication of cerebral angiography. The incidence was reported to be 0.3-1% of cerebral angiography [1]. With newer contrast agents, the incidence of 0.2% was reported recently [2]. Its pathophysiology remains uncertain. Patients complained of cortical blindness within minutes of contrast injection and usually resolved within few days [1]. It is reported to have a higher incidence for vertebral angiography [3]. We would like to report a case of TCB following vertebral angiography with multimodality imaging performed.
CASE REPORTS
A 57-years-old male with good past health was presented to emergency department of our hospital on 2nd June 2013, complained of sudden onset of severe headache and vomiting without history of head injury. Patient was all along conscious with GCS 15/15 and no focal neurological deficit was elicited on physical examination. Urgent non-contrast CT showed diffuse acute subarachnoid haemorrhage (SAH). Diagnostic cerebral angiograms were performed on the next day. A 0.7 cm narrow-neck aneurysm was identified over the origin of left posterior inferior cerebellar artery (PICA). Coil embolization of the left PICA aneurysm was performed one day later. Small residual neck was left in order to preserve origin of left PICA. No immediate complication was noted. Patient showed good recovery with no vasospasm and was discharged on day14 post-SAH.
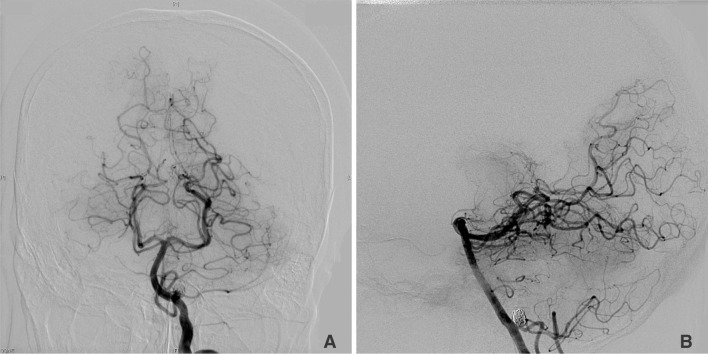
Elective follow-up left vertebral angiogram was performed 4 months later with right femoral approach and selective catheterization of left vertebral artery using 5Fr headhunter type catheter. The contrast agent used was Omnipaque 300 mg/ml and 20 ml of contrast was used in total. Patient then developed bilateral blindness immediately after angiography. Patient remained normotensive during the angiography. Review of angiograms showed no sign of vascular occlusion or spasm in the vertebrobasilar territory (Fig. 1).
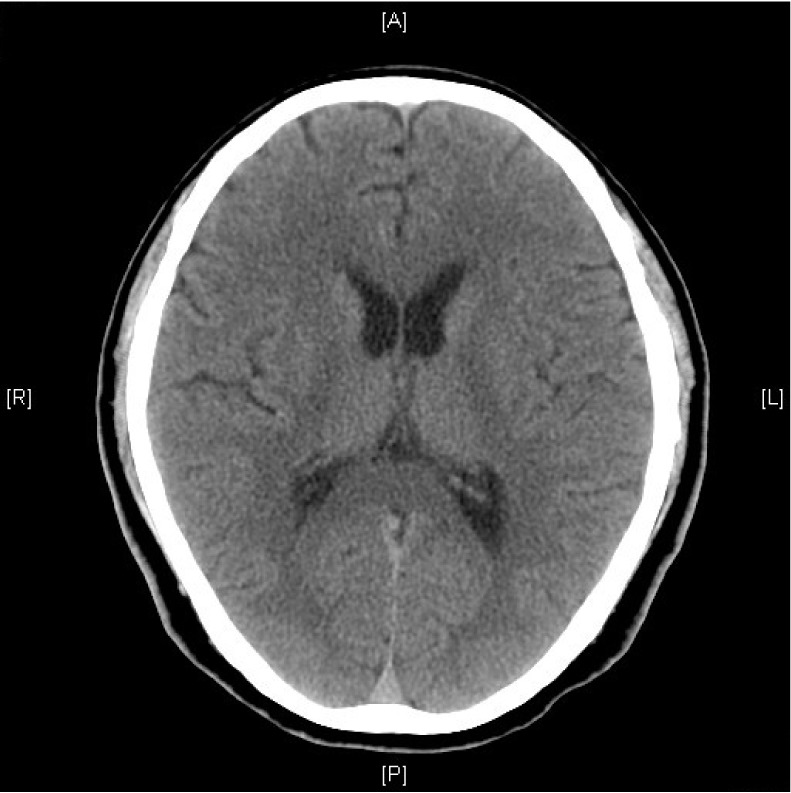
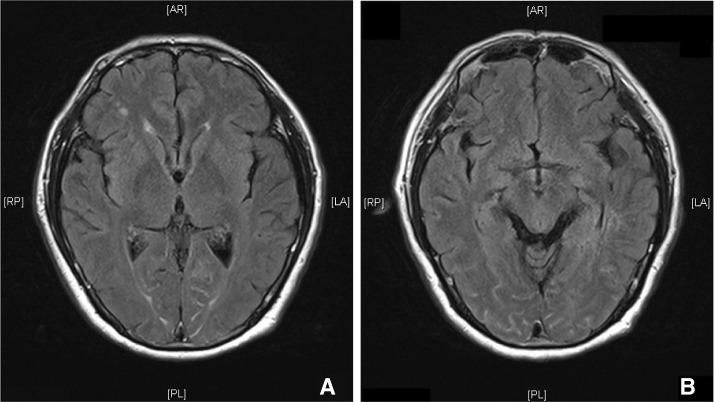
Urgent non-contrast brain CT was performed within 2 hours after angiography and showed no evidence of acute infarction over bilateral parieto-occipital cortex (Fig. 2). Brain MRI was arranged within 3 hours after angiography and demonstrated no abnormality on T1-weighted, T2-weighted, and post-gadolinium T1-weighted images. Post-gadolinium FLAIR MR images showed abnormal high signal over sulcal spaces of bilateral parieto-occipital lobes (Fig. 3). No restricted diffusion was seen on diffusion weighted images and apparent diffusion coefficient (Fig. 4). Assessment by an ophthalmologist revealed no local cause of sudden visual loss. Short course of steroid was given. Patient's vision was normalized within 24 hours after vertebral angiography. Patient was discharged one week later with no residual neurological deficit.
DISCUSSION
TCB is a rare but dramatic complication of cerebral angiogram. More than 600 cerebral angiograms were performed in our hospital over the past 5 years and this is our first case of TCB following cerebral angiogram (0.16%).
The exact mechanism of TCB following cerebral angiography is unknown and there are two main hypotheses. The first hypothesis is that it is a result of the neurotoxic effect of contrast agent causing osmotic disruption of blood-brain barrier [4]. This hypothesis is supported by the different sympathetic innervation of posterior circulation, which is more susceptible to such injury [5]. The contrast agent used in our reported case was Omnipaque, a non-ionic contrast agent. When used without dilution, it is hyperosmotic to blood, contributing to disruption of blood-brain barrier. Reported MRI findings of blood-brain barrier disruption The second hypothesis is that TCB is the result of posterior reversible leukoencephalopathy syndrome (PRES) [6], which is due to failure of cerebrovascular autoregulation, leading to perivascular edema. Typical MRI features of PRES is subcortical distribution of high signal in FLAIR and DWI in parieto-occipital regions [7]. In our case, abnormal high signal intensity was noted in the sulcal spaces of bilateral parieto-occipital lobes on post-gadolinium FLAIR images, not in the subcortical white matter. No abnormal signal was detected on T1, T2 and post-gadolinium T1-weighted images. To our knowledge, these findings have not been described in patients with TCB previously in the literature. This finding may support the hypothesis of blood-brain barrier disruption by contrast neurotoxicity in patients with TCB. Subarachnoid high signal intensities on FLAIR images can be related to blood and exudates with high protein content. Post-gadolinium FLAIR has been suggested to be more sensitive for detecting abnormal signal intensity in subarachnoid spaces than post-gadolinium T1-weighted sequence [8]. In the case of blood-brain barrier dysfunction, leakage of gadolinium into extracellular space may shorten the T1 value causing FLAIR high signal intensity in the subarachnoid spaces.
In the clinical context of sudden blindness after cerebral angiography, one should differentiate TCB from embolic infarction as a complication of angiography. Bilateral blindness is more likely to be related to TCB rather than embolism affecting both occipital lobes which has been reported after cardiac catheterization though this is very rare [9]. The use of MRI is helpful in this context as there is no restricted diffusion of the affected occipital lobes in case of TCB as in our case, while there will be restricted diffusion in cases of acute infarction related to cerebral embolism.
The treatment of these two conditions are different as more active thrombolytic therapy may be needed in case of ischemic infarct, while for TCB no treatment is needed because it is self-limiting and reversible. In fact, no treatment has been proven to be effective to improve the natural history of TCB following cerebral angiography [6,10]. It usually lasts from a few hours to a few days. Patients should be reassured that symptoms will resolve spontaneously within few days.
In summary, we reported a case of TCB following vertebral angiography, a rare but dramatic complication of cerebral angiography. With our imaging finding of sulcal high signal intensity on post-gadolinium FLAIR images, the mechanism is more likely related to neurotoxicity of contrast agent injected during angiography with transient disruption of blood-brain barrier. Radiologists and clinicians should be aware of this complication. When it occurs, one should reassure the patient about the excellent prognosis of this condition.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download