This article has been
cited by other articles in ScienceCentral.
Abstract
The PulseRider is a neuroendovascular adjunct for wide-necked intracranial aneurysms. The decreased metal burden of the PulseRider theoretically reduces artifact on radiologic imaging. However, we report here on a case of a patient who underwent PulseRider-assisted stent-coiling of a basilar tip aneurysm. He returned 19 months later for intermittent diplopia and darkening of vision but was neurologically intact on exam. Both contrast-enhanced and time-of-flight magnetic resonance angiography (MRA) demonstrated absence of signal in the basilar artery in the proximal anchors of the PulseRider. Given his lack of reproducible symptoms and high functional status, it is presumed that the imaging reflected artifact and not thrombosis/stenosis. Although the PulseRider is a useful treatment option for wide-necked intracranial aneurysms, the clinician should be aware that even contrast-enhanced MRA can produce artifact that resembles thrombosis/stenosis. Non-angiogram radiologic imaging modalities may be appropriate for evaluation for residual aneurysm but not patency of the parent artery.
Keywords: PulseRider, Intracranial aneurysm, Stent-assisted coiling, Neuroendovascular surgery, Neurosurgery
INTRODUCTION
Wide-necked intracranial aneurysms are traditionally treated by open microsurgical clipping, primary or stent-assisted coiling, or balloon remodeling [
1]. A number of different treatments have been developed to accommodate variations in anatomy. A risk of traditional coiling of wide-necked aneurysms is migration of the coils back into the parent artery, and traditional stent-assisted coiling of basilar tip aneurysms is not optimal due to the relationship of the aneurysm to the posterior cerebral arteries. Stent-assisted coiling of basilar tip aneurysms has been described with placing two stents in a Y-shaped configuration or by placing a horizontally oriented stent, but the technical difficulty of these approaches has incentivized the design of adjunct devices such as the PulseRider.
The PulseRider (Cerenovus, Miami, FL, USA) is a neuroendovascular adjunct that allows for a variation on stent-assisted coiling. It is mostly made of nitinol with platinum/iridium alloy radiopaque markers and also contains stainless steel at the proximal detachment points. The PulseRider can treat bifurcation aneurysms that have a branch artery angle of up to 120 degrees relative to the parent artery. Endovascular treatment of widenecked cerebral aneurysms with the PulseRider has a high technical success rate, and there is complete occlusion of approximately two-thirds of aneurysms at one-year after treatment and adequate occlusion of 90% at one-year [
2,
3]. Additionally, the PulseRider is composed of less metal than a traditional stent with larger fenestrations, which may allow for easier coiling.
Follow-up of patients who received the PulseRider can be conducted with conventional cerebral digital subtraction angiography (DSA), magnetic resonance angiography (MRA), or computed tomography angiography (CTA) per the Federal Drug Administration (FDA) instructions for use. We report on the case of a contrast-enhanced MRA (CE-MRA) of a PulseRider-treated patient demonstrating focal absent flow signal at the proximal end of the PulseRider due to device artifact.
CASE REPORT
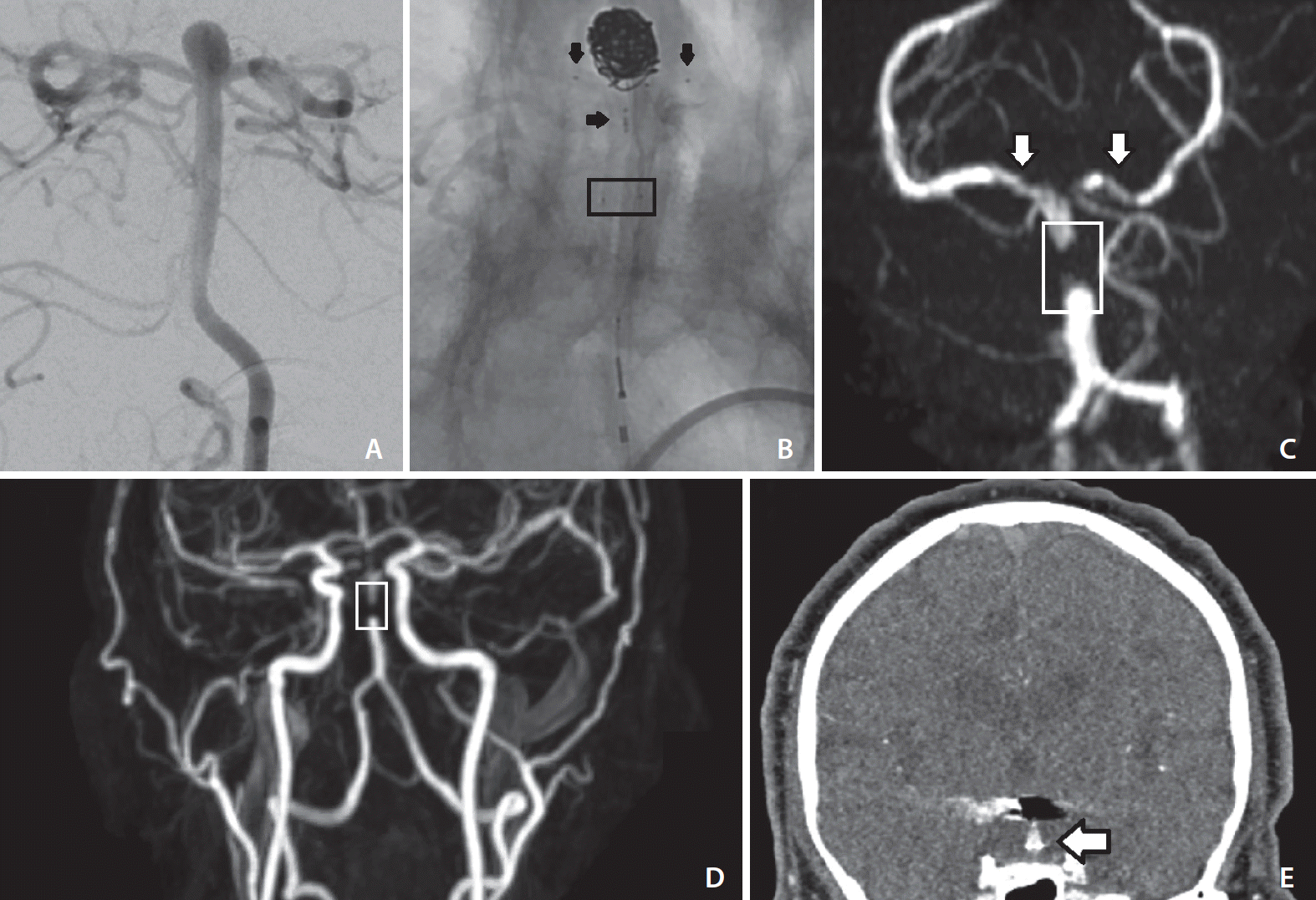
An elderly patient presented to clinic for neurosurgical evaluation of an expanding basilar tip aneurysm. The aneurysm measured 7 mm in height with a neck of 4 mm in width (
Fig. 1A). Given the location and wide-necked configuration of the aneurysm, endovascular treatment was favored, and traditional coiling would not be sufficient. After discussion of risks and benefits, the patient elected to proceed with PulseRider-assisted coiling. A 211-D (parent vessel diameter 2.7–3.5 mm) T-shaped PulseRider was successfully deployed with the T-struts well-aligned to the P1 segments of the posterior cerebral arteries (
Fig. 1B). The patient was discharged within 24 hours with instructions to take aspirin 81 mg and clopidogrel 75 mg daily. The patient did not return for scheduled postoperative clinic; however, at 19-months following surgery he returned to his primary physician for episodic diplopia (including monocular vision diplopia) and intermittent darkening of vision that occurred approximately twice per month.
CTA head obtained at that time was unremarkable, although the study was degraded by metallic streak artifact from the PulseRider and coils. He was referred back to our clinic for evaluation. At 20-months after surgery, time-of-flight MRA (TOF-MRA) of the head and CE-MRA of the neck (both 3 Tesla, echo time 22, echo train length 27, repetition time 700, flip angle 120) were performed. Both the modalities demonstrated lack of signal within the junction of the middle and distal thirds of the basilar artery trunk, in the vicinity of the detachment zone of the PulseRider device (
Fig. 1C,
D). However, both imaging modalities demonstrated flow within the distal basilar artery at the bifurcation to the posterior cerebral arteries. Thus, the artifact was limited to the anchors. Of note, there was no evidence of residual or recurrent aneurysm. Comparison against the patient’s prior CTA demonstrated that this segment of the basilar artery produced significant metal artifact (
Fig. 1E). The patient’s symptoms were not reproducible, and he was neurologically intact. Given the patient’s reassuring neurologic exam and the imaging findings, it was determined that the aneurysm was successfully treated and that the loss-of-signal was artifact. The vision complaints prompting this re-evaluation at 20-months were not due the treated aneurysm or the surgery. DSA was offered to the patient to rule out absence of in-stent stenosis or evaluate for other possible cerebrovascular causes of the patient’s symptoms, but the patient declined the procedure.
DISCUSSION
In this case report, we describe and demonstrate CE-MRA artifact due to the PulseRider device. The patient was followed up with a CE-MRA that demonstrated a focal loss of both TOF and contrast-enhanced flow signal along a portion of the proximal margin of the PulseRider, raising concern for device-related vessel stenosis or thrombus formation. The PulseRider FDA instructions for use state that DSA, MRA, or CTA are appropriate modalities for follow-up. Large multicenter studies have allowed for the treating surgeon to follow with their imaging modality of choice [
2,
3]. This case report implies that MRA is appropriate for follow-up aneurysms but not necessarily the parent vessel.
A literature review of the PubMed Central database was performed to review cases of PulseRider-treated follow-up imaging. This was performed by utilizing the search terms (“PulseRider” OR “Pulse Rider”). The literature review returned 44 works. Excluding conference poster and oral presentations, 37 articles were evaluated. All articles were then reviewed for demonstration or mention of artifact on follow-up MRA after PulseRider treatment of intracranial aneurysms. One study reported artifact and non-visualization of the parent artery with TOF-MRA [
4]. Another study demonstrated similar artifact on MRA but does not specify the exact MRA modality [
5]. However, the authors of this study do cite another study that investigates optimization of TOF-MRA when mentioning this artifact. While Mukherjee et al. [
6] mention that follow-up magnetic resonance imaging demonstrates stent artifact, they do not specify the exact modality or demonstrate the artifact, and their statement implies that magnetic resonance modalities cannot be utilized to evaluate the aneurysm itself. They do not mention evaluation of the parent vessel. No study demonstrated loss of signal with CE-MRA.
CE-MRA has been compared to TOF-MRA, with DSA as the gold standard comparison, for evaluation of aneurysms following treatment with Pipeline Embolization Device, coils, and stent-assisted coils [
7-
9]. CE-MRA has been demonstrated to better visualize stented arteries, reduce coil artifact, and more accurately measure luminal diameter. CE-MRA correlates better with DSA than TOF-MRA [
10]. Outside of the literature review, non-visualization of a parent artery in a PulseRider-treated patient on TOF-MRA was corrected with CE-MRA [
11].
This is the first description in conjunction with radiographic depiction of a focal artifact in the proximal portion of the PulseRider device observed on CE-MRA. This is likely due to the stainless steel that is retained at the detachment points as the “marker band effect” previously described in the literature for nitinol Neuroform (Stryker, Fremont, CA, USA) stents occurred at vessels less than 2mm thick, and the Neuroform markers lie in a plane perpendicular to the flow of blood whereas the PulseRider markers are parallel [
12]. Stainless steel, on the other hand, has been demonstrated to cause complete loss of signal even in a stent that is 8 mm thick [
13]. The artifact demonstrated here does not hinder evaluation of residual or recurrent aneurysm as there is distal reconstitution of signal after a short segment of artifact. The PulseRider remains a useful adjunct, but clinicians should be aware of this possibility on postoperative imaging.
Although this work is limited in that it is a single case report, we believe it is important for neuroendovascular surgeons to be aware that focal loss of signal within the parent artery can radiographically appear as an in-stent stenosis or thrombosis and this artifact can persist even with CE-MRA in patients with the PulseRider. Thus, while MRA may be a useful imaging modality for assessment of residual aneurysm, it may be limited in assessment of the patency of the parent artery. While there is the remote possibility this patient did indeed have an in-stent stenosis, this is considered unlikely given that his symptoms had a functional overlay component, and he had no persistent neurologic deficits on exam. However, this is a possible limitation of this case report. The patient followed up with physicians outside of our system, so to our knowledge there is no established diagnosis for the patient’s symptoms. However, given that he was not referred back to us, there was presumably not an identified cerebrovascular cause for the patient’s symptoms. Further studies will be necessary to determine how frequently this artifact occurs, especially considering the overall lower metal burden of the PulseRider.
The PulseRider is a newer FDA-approved adjunctive device that can be used for the treatment of wide-necked intracranial aneurysms. Follow-up imaging of patients who undergo endovascular treatment with the PulseRider can be conducted by DSA, MRA, or CTA. Although CE-MRA has been demonstrated to be better than TOF-MRA for follow-up of aneurysms treated with devices with metal artifact, CE-MRA may still fail to resolve artifact produced in the parent artery of a wide-necked aneurysm treated by PulseRider-assisted coiling and thus may be most appropriate for evaluation for residual aneurysm but not patency of the parent artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download