Abstract
Purpose
Materials and Methods
Results
Conclusion
Notes
Ethics Statement
The study was approved by the Institutional Research Ethics Board (REB# 1000056549). Written informed consent was obtained from all patients/parents for publication before the procedures. Need for individual consent for this retrospective review was waived by the REB.
Author Contribution
Concept and design: AAD, KB, and PM. Analysis and interpretation: AAD, WH, SB, MS, and PM. Data collection: AAD, WH, and PM. Writing the article: AAD, WH, KB, and PM. Critical revision of the article: AAD, SB, MS, PD, and PM. Final approval of the article: AAD, PD, and PM. Statistical analysis: AAD, SB, KB, and PM. Obtained funding: AAD and SB. Overall responsibility: AAD, KB, and PM.
REFERENCES
Fig. 1.
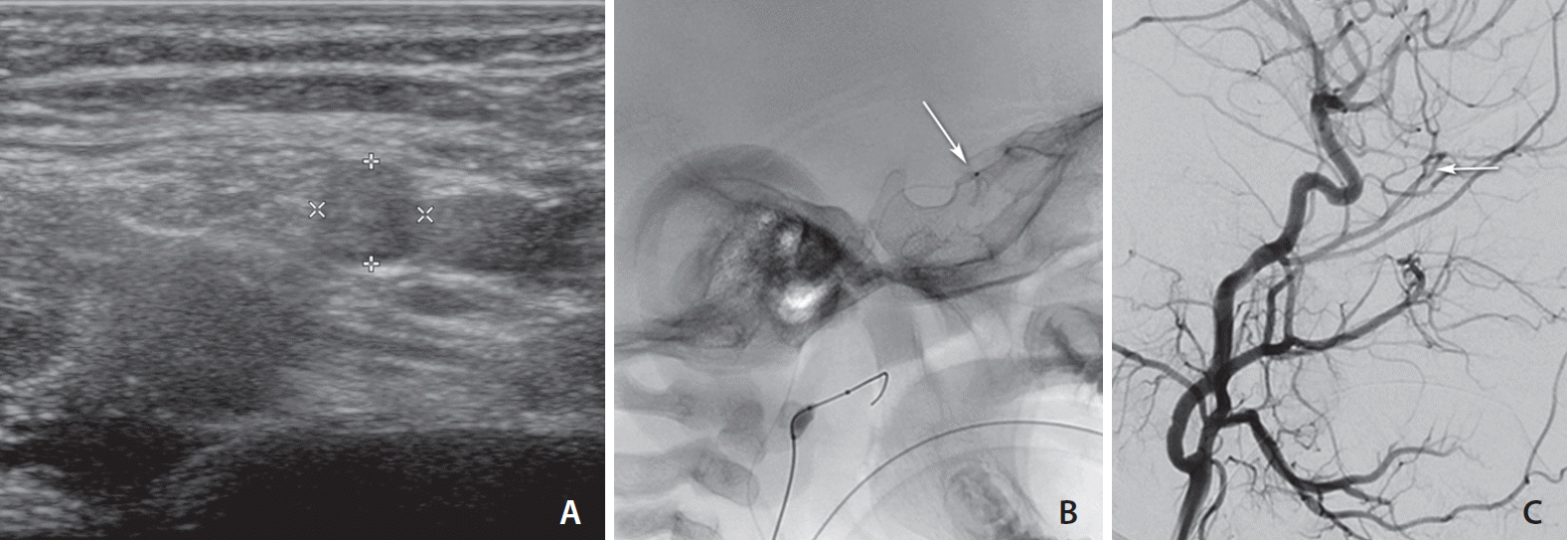
Fig. 2.
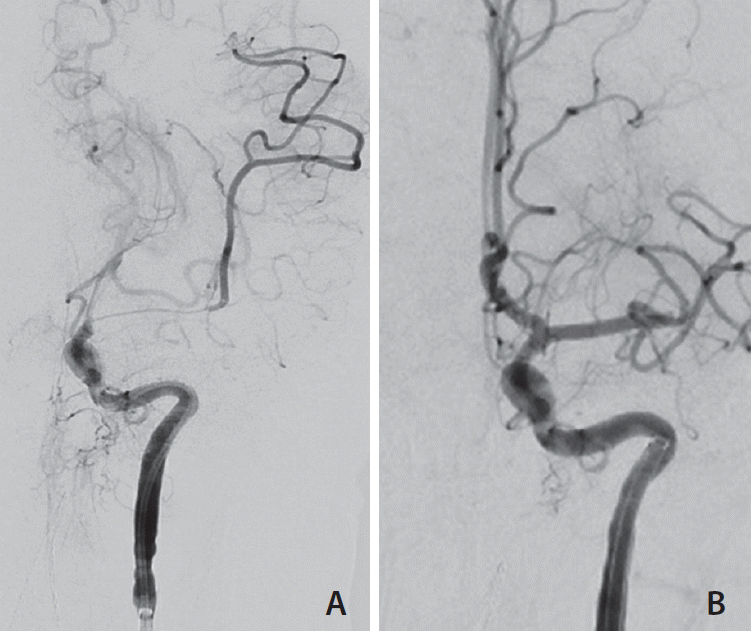
Table 1.
Table 2.
Table 3.
| Variable | All procedures (n=27) | ≤15 kg procedures (n=9) | >15 kg procedures (n=18) |
|---|---|---|---|
| Reason for long sheath | |||
| Dual microcatheter technique | 14 (51.9) | 6 (66.7) | 8 (44.4) |
| Triaxial support | 9 (33.3) | 3 (33.3) | 6 (33.3) |
| Flow reversal | 3 (11.1) | 0 (0) | 3 (16.7) |
| Intra-operative angiography | 1 (3.7) | 0 (0) | 1 (5.6) |
| Procedure | |||
| Embolization | 13 (48.1) | 1 (11.1) | 12 (66.7) |
| Embolization of shunting lesions | 9 (33.3) | 1 (11.1) | 8 (44.4) |
| Tumor embolization | 2 (7.4) | 0 (0) | 2 (11.1) |
| Aneurysm treatment | 1 (3.7) | 0 (0) | 1 (5.6) |
| Pseudoaneurysm embolization | 1 (3.7) | 0 (0) | 1 (5.6) |
| Intra-arterial chemotherapy | 10 (37.0) | 8 (88.9) | 2 (11.1) |
| Mechanical thrombectomy for stroke | 3 (11.1) | 0 (0) | 3 (16.7) |
| Diagnostic angiography (intra-operative) | 1 (3.7) | 0 (0) | 1 (5.6) |
| Sheath/Catheter used without short sheath | |||
| 5 Fr Flexor Shuttle sheath (ID 0.074 inch) | 16 (59.3) | 8 (88.9) | 8 (44.4) |
| Penumbra Benchmark intracranial access catheter (OD 6 Fr, ID 0.071 inch)* | 5 (18.5) | 0 (0) | 5 (27.8) |
| 6 Fr Penumbra Neuron Max sheath (ID 0.088 inch) | 4 (14.8) | 0 (0) | 4 (22.2) |
| 6 Fr Flexor Shuttle sheath (ID 0.087 inch) | 1 (3.7) | 1 (11.1) | 0 (0) |
| Microvention Chaperon guiding catheter (OD 6 Fr, ID 0.071 inch) | 1 (3.7) | 0 (0) | 1 (5.6) |
| Vessel accessed | |||
| Internal carotid artery | 10 (37.0) | 7 (77.8) | 3 (16.7) |
| Common carotid artery | 9 (33.3) | 0 (0) | 9 (50.0) |
| Brachiocephalic | 2 (7.4) | 1 (11.1) | 1 (5.6) |
| Left vertebral artery | 2 (7.4) | 0 (0) | 2 (11.1) |
| Right Subclavian Artery | 2 (7.4) | 0 (0) | 2 (11.1) |
| Aorta | 1 (3.7) | 0 (0) | 1 (5.6) |
| Left Subclavian | 1 (3.7) | 1 (11.1) | 0 (0) |
| Heparin use | |||
| Yes | 25 (92.6) | 9 (100) | 16 (88.9) |
| Mean procedural heparin dose (U/kg) | 101.7±38.0 | 98.7±4.0 | 103.3±47.9 |
| Ultrasound guidance for access | 27 (100) | 9 (100) | 18 (100) |
| Mean fluoroscopy time (min) | 62.0±43.1 | 45.8±39.1 | 70.6±43.7 |
| Mean procedure time (h) | 4.5±2.2 | 4.0±1.2 | 4.9±2.8 |
| Post-procedural hemostasis | |||
| Manual pressure | 25 (92.6) | 8 (88.9) | 17 (94.4) |
| Closure device | 2 (7.4) | 1 (11.1) | 1 (55.6) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download