This article has been corrected. See "Fund Correction: Unrecognized Ruptured Intracranial Aneurysm Presenting as Cerebral Vasospasm-Induced Ischemic Stroke: A Case Report" in Volume 16 on page 304.
Abstract
A 44-year-old woman presented with acute confusion, apparently due to a clinically silent subarachnoid hemorrhage followed by vasospasm, which in turn led to an ischemic stroke. During the initial evaluation, an acute ischemic stroke in the left middle cerebral artery territory was observed. Magnetic resonance imaging revealed a late subacute hemorrhage in the left basal cistern. Digital subtraction angiography indicated the presence of a small saccular aneurysm that had recently ruptured, as well as vasospasm in the left circle of Willis. Balloon angioplasty and balloon-assisted coil embolization were performed for the vasospasm and saccular aneurysm, respectively. This case demonstrates that clinically silent subarachnoid hemorrhages resulting in ipsilateral vasospasm and infarction can occur as complications of a ruptured aneurysm.
The most distinctive clinical manifestation of subarachnoid hemorrhage (SAH) related to a ruptured aneurysm is a thunderclap headache, which may be accompanied by focal neurological deficits, and/or decreased levels of consciousness [1]. However, if the clinical symptoms are vague or absent, even careful evaluation may result in the misdiagnosis of SAH during the initial admission [2]. Especially, if the patient’s symptoms and neuroimaging results are compatible with those of acute ischemic stroke, prompt and accurate diagnosis of cerebral vasospasm by ruptured aneurysm may be challenging [2-4]. A minor leakage prior to major subarachnoid hemorrhage without headache is rare and, if unrecognized, is associated with devastating clinical outcomes [5]. In this case report, we describe a patient who visited the hospital with symptoms of ischemic stroke, which were likely secondary to delayed cerebral vasospasm arising from an unrecognized minor leak of aneurysm several days prior.
A 44-year-old right-handed woman presented with sudden-onset confusion and disorientation. She had been involved in two mild car accidents on the day of hospital admission. She was transferred to the emergency room after she spoke confused words to a police officer investigating the second car accident. An initial neurological examination conducted in the emergency room revealed mild motor aphasia and right-hand clumsiness.
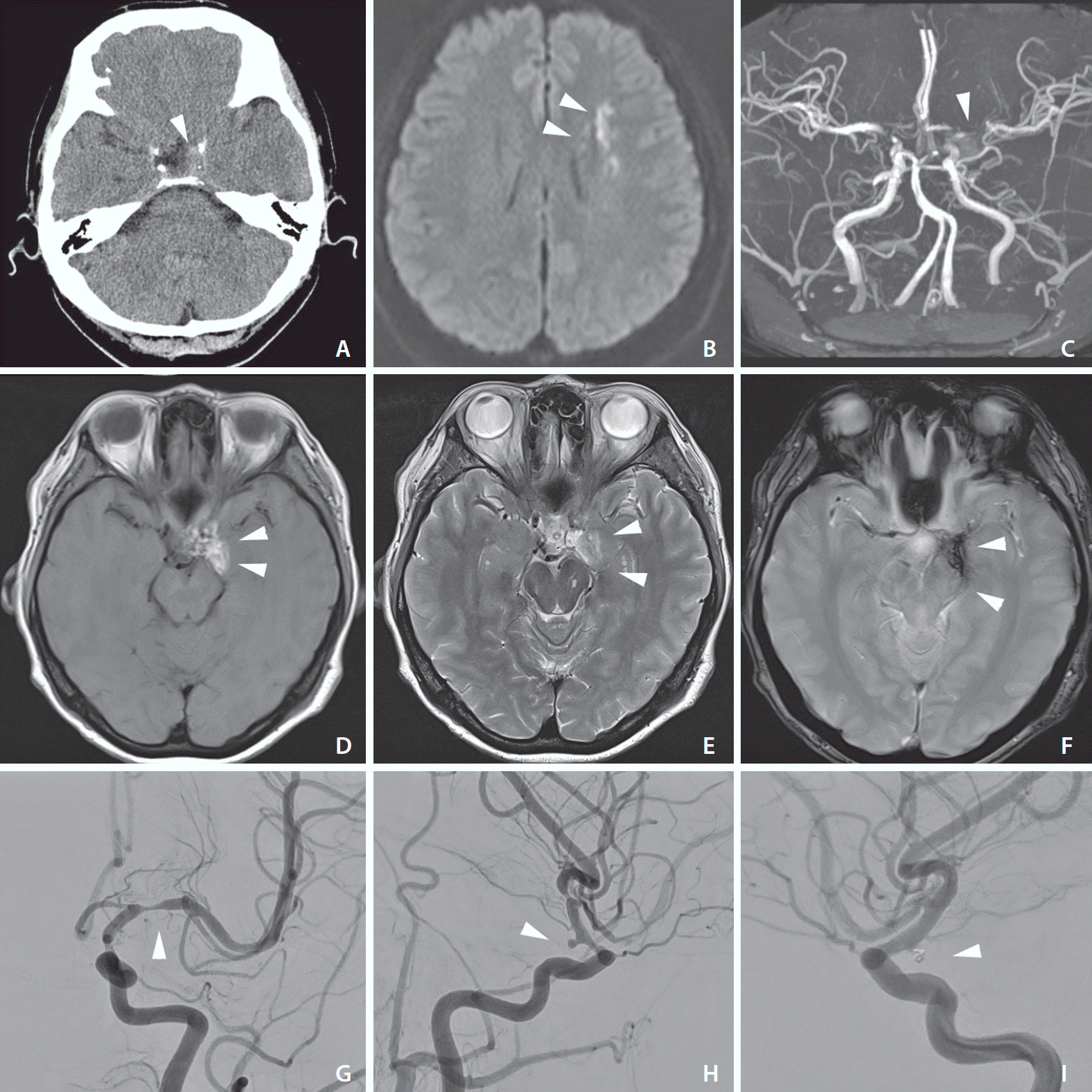
A computerized tomography (CT) scan of the brain revealed a small hyperattenuating lesion, mainly in the lower basal cistern surrounding the base of the patient’s skull (Fig. 1A). In initial magnetic resonance imaging (MRI), diffusion-weighted imaging of the brain revealed tiny, scattered high-intensity lesions compatible with ischemic stroke in the left middle cerebral artery (Fig. 1B). Additionally, axial T1- and T2-weighted imaging and low gradient-echo T2 image showed high signal intensity in the left basal cistern that indicating a late subacute hemorrhage (Fig. 1D–F). However, Initial magnetic resonance angiography (MRA) showed severe stenosis of the left middle cerebral artery but did not clearly demonstrate the aneurysm. (Fig. 1C). The digital subtraction angiography (DSA) revealed the presence of a left posterior communicating artery (P-ComA) aneurysm, with severe stenosis in the ipsilateral distal internal carotid and middle cerebral arteries (Fig. 1G, H). Accordingly, the ischemic stroke was shown to be related to delayed cerebral vasospasm caused by a minor leak of impending aneurysm rupture.
The patient was transferred to the department of neurosurgery. An anteroposterior and lateral angiograph confirmed severe spasms of the left middle cerebral artery. Heparin and nimodipine mixed in normal saline were infused continuously into the cervical internal carotid artery through an Envoy 6-French (Fr) guiding catheter (Codman, Raynham, MA, USA). For balloon angioplasty, micro-balloon catheters (Scepter C 4.0×11 mm; MicroVention Terumo, Tustin, CA USA) were advanced over a Synchro microwire into the left proximal M1 segment of MCA. The post-treatment angiography confirmed the resolution of the vasospasm, and the caliber of the left middle cerebral artery was markedly improved. Coil embolization was simultaneously performed for the left P-ComA aneurysm. Initially, the standard technique was attempted, but the coil protruded into the parent artery. Thus, we decided to treat the aneurysm using a balloon-assisted technique. The balloon angioplasty was performed in the same manner as the vasospasm in the left clinoid segment of the internal carotid artery. The final angiogram showed that the aneurysm was nearly completely obliterated, with minimal neck remnant and no complications. The patient was discharged without any residual neurological deficits. A follow-up DSA performed one year after the endovascular treatment revealed no significant changes in the tiny aneurysmal neck remnant, and the parent artery was well-preserved with no stenosis (Fig. 1I).
The cerebral vasospasm following SAH may result in ischemic stroke indistinguishable on initial evaluation from the other cause of ischemia [3,6]. Thus, the initial clinical manifestation of the patient, such as a headache that preceding SAH by days is given critical information for early diagnosis [2,7]. Nussbaum et al. [8] reported the two cases of intracranial aneurysm rupture present as delayed stroke secondary to cerebral vasospasm. All of these patients complained to their families about the sudden onset of headache several days before the diagnosis. However, the distinguishable point of our case compared with other reports was the absence of headaches. The previous reports showed that between 2% to 8% of patients with SAH do not present headaches [5]. Although the cause of the missing headache symptom and the association with left-sided aneurysm location is unclear, perhaps left hemisphere dysfunction could potentially lead to aphasia or lack of attention on the part of the patient when the patient describes symptoms to a physician. Thus, our report emphasized the need to recognize and identify the correct diagnosis of SAH even without headache. If the amount of bleeding is extremely small, patients with initial good neurologic grades are at high risk of being misdiagnosed. Brain CT remains relatively less sensitive to SAH more than a few days old, especially in cases where the bleed is small [6,7]. Initial MR angiography of this patient demonstrated the middle cerebral artery stenosis, which challenging to discriminated from cerebral vasospasm associated with a SAH. Also, only DSA could be revealed an aneurysm missed on initial MR angiography. Initial MR time of flight (TOF) images missed aneurysm due to poor resolution and visualization, which seemed to be related to vasospasm and hemorrhage. Additionally, if the initial neuroimaging suggests compatible findings with acute ischemic stroke, subarachnoid hemorrhage would be easily underdiagnosed in a patient with suddenly developed neurologic deficit.
In patients presenting with ischemic stroke due to vasospasm secondary to a minor leak of a ruptured aneurysm, the clinical manifestations may be similar to other stroke subtypes [8]. Under/Mis-diagnosis at the initial hospital visit has been correlated with a rise in the re-bleeding risk, clinical deterioration, and catastrophic outcome. However, making a precise diagnosis of SAH without the typical symptoms, especially headache, is challenging. However, the therapeutic approach to vasospasms is completely different from that for cerebral ischemia of other etiologies [9]. The treatment should be aimed at preventing aneurysm rebleeding and restoration of the arterial narrowing responsible for the ischemic symptoms. In addition to balloon angioplasty, anti-spasmodic agents such as nimodipine or verapamil can be infused intra-arterially to resolve the vasospasm. Conventional triple-H therapy (Hypertension, hypervolemia, and hemodilution) is often utilized to prevent and treat cerebral vasospasm after SAH [10]. However, confirmation of leakage from an undiagnosed ruptured intracranial aneurysm is most crucial in the initial approach [4]. A small leak that precedes recurrent catastrophic bleeding is common and associated with high mortality [2]. Surgical or endovascular obliteration of an aneurysm is particularly important because the risk of rebleeding within days or weeks if the ruptured aneurysm is left untreated is 25–75% [5].
CT is beneficial for detecting acute SAH, but its utility may be limited in cases of poor-grade, infratentorial, or subacute SAH [4,7]. If the subacute hemorrhage is small and localized within the skull base, even expert physicians can misdiagnosis. Thus, T1, T2, FLAIR, and gradient echo(or Susceptibility weighted imaging) MRI sequences have been developed [11]. These advanced sequences significantly improve the power of MRI for detecting late subacute SAHs, such as in this case. A combination of these MRI sequences is proven to benefit in cases with inconclusive CT scans of suspected minor leaks of aneurysms [3,4]. Nevertheless, routine MRA is unreliable for identifying cerebral aneurysms less than 5 mm in size [12]. Three-dimensional time-of-flight MRA was affected by the residual SAH and vasospasm and failed to reveal the aneurysm in our patient. Thus, DSA remains the gold standard for detecting tiny aneurysms despite a negative MRA; it can also confirm vasospasms, which may be candidates for revascularization [6].
In this patient, given the aneurysm location and its parent vessels’ shape, differential diagnosis from a blood blister-like aneurysm (BBA) was necessary. BBAs may be misdiagnosed as saccular aneurysms of the ophthalmic, anterior choroidal, or P-ComA arteries [13]. However, given that the hemorrhage was localized around the left basal cistern in this case and that the late subacute stage hemorrhage was confirmed on MRI, the irregular vessel appearance was more likely related to vasospasm than a BBA.
In conclusion, cerebral vasospasm resulting from minor leaks due to an unrecognized cerebral aneurysm rupture is rare. Still, it is a possible cause of ischemic stroke requiring close attention and proper treatment. The importance of establishing a precise diagnosis cannot be overstated because of the risk of rebleeding and because misuse of antithrombotic therapy may have grave consequences, such as in this case. Thus, it may be prudent to perform a DSA conventional angiography to rule out SAH due to an unrecognized ruptured aneurysm if any doubt remains after reviewing the clinical history or CT and MRI/MRA scans.
Notes
REFERENCES
1. Heros RC. Intracranial aneurysms. A review. Minn Med. 1990; 73:27–32.
2. Kowalski RG, Claassen J, Kreiter KT, Bates JE, Ostapkovich ND, Connolly ES, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004; 291:866–869.

4. Oda S, Shimoda M, Hirayama A, Imai M, Komatsu F, Shigematsu H, et al. Neuroradiologic diagnosis of minor leak prior to major SAH: diagnosis by T1-FLAIR mismatch. AJNR Am J Neuroradiol. 2015; 36:1616–1622.

5. Kassell NF, Torner JC. Aneurysmal rebleeding: a preliminary report from the cooperative aneurysm study. Neurosurgery. 1983; 13:479–481.

6. de Oliveira Manoel AL, Mansur A, Murphy A, Turkel-Parrella D, Macdonald M, Macdonald RL, et al. Aneurysmal subarachnoid haemorrhage from a neuroimaging perspective. Crit Care. 2014; 18:557.

7. Davis KR, Kistler JP, Heros RC, Davis JM. A neuroradiologic approach to the patient with a diagnosis of subarachnoid hemorrhage. Radiol Clin North Am. 1982; 20:87–94.
8. Nussbaum ES, Sebring LA, Wen DY. Intracranial aneurysm rupture presenting as delayed stroke secondary to cerebral vasospasm. Stroke. 1997; 28:2078–2080.

9. Adams HP Jr, Jergenson DD, Kassell NF, Sahs AL. Pitfalls in the recognition of subarachnoid hemorrhage. JAMA. 1980; 244:794–796.

10. Bauer AM, Rasmussen PA. Treatment of intracranial vasospasm following subarachnoid hemorrhage. Front Neurol. 2014; 5:72.

11. Wiesmann M, Brückmann H. [Magnetic resonance imaging of subarachnoid hemorrhage]. Rofo. 2004; 176:500–505. German.
Fig. 1.
(A) There is a subtle high density within the basal cistern—just lateral to the medial temporal lobe (white arrowhead). There is minimal, if any localized edema within the region of hemorrhage. No further evidence of blood spread out from the basal cistern. (B) High signal intensity on diffusion-weighted images indicated an acute ischemic stroke in the left middle cerebral artery territory (white arrowheads). (C) Three-dimensional time-of-flight magnetic resonance angiography revealed stenosis of the middle cerebral artery (white arrowhead). (D) Axial T1 weighted imaging shows conspicuous bright lesions, including the neighboring area of the left basal cistern (white arrowheads). (E) T2-weighted imaging shows high signal intensity in the left basal cistern, implying a late subacute hemorrhage (white arrowheads). (F) Subarachnoid hemorrhage was detected on the gradient echo T2 image as an area of low signal (white arrowheads). (G) The digital subtraction angiography revealed severe stenosis (white arrowhead), indicating vasospasm of the left middle cerebral artery. (H) Lateral angiography confirmed the presence of a left posterior communicating artery aneurysm (white arrowhead) along with parent artery narrowing. (I) The saccular aneurysm was treated using a balloon-assisted coil embolization technique (white arrowhead).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download