Abstract
Flow diversion stenting combined with coiling offers both immediate protection from rebleeding for ruptured aneurysms and long-term stability for wide-necked or blister aneurysms. It is particularly useful for tiny ruptured aneurysms, alleviating the concern that small coils may prolapse between the struts of conventional stents. We employed this technique in a very small, broad-based ruptured aneurysm of the internal carotid, jailing the coiling microcatheter with a Pipeline Embolization Device. However, coil detachment repeatedly failed, until we withdrew the detachment zone into the microcatheter. We suggest that if the tip of the coiling catheter is adjacent to the stent, contact between the junction zone of the coil and the high metal density of the flow diverter may prevent proper electrothermal coil detachment. Detachment can be undertaken successfully within the microcatheter, though care must be taken thereafter to fully push the detached coil tail into the aneurysm.
Failure of coil detachment is a serious problem in any neurointerventional case, but particularly so in technically complex cases. This case identifies a potential technical problem in endovascular management of tiny blister aneurysms, and offers a solution that may be useful to others facing this situation.
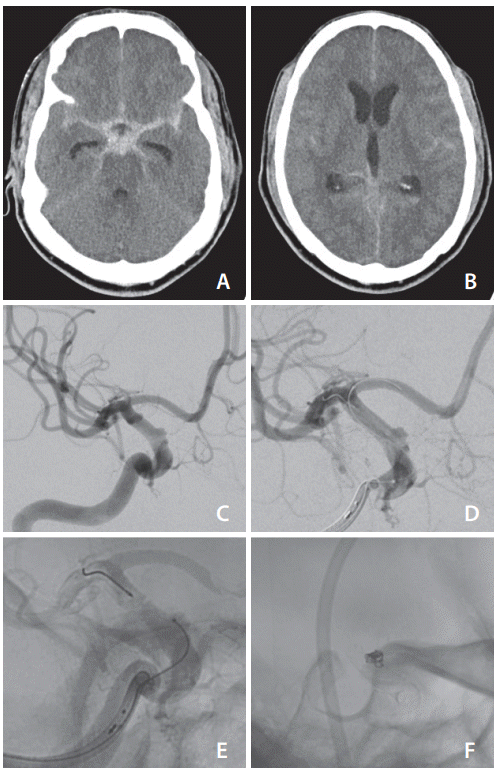
A previously healthy patient in their 50s presented with sudden onset severe headache and confusion. Computed tomography (CT) confirmed extensive diffuse subarachnoid hemorrhage and moderate hydrocephalus (Fig. 1A, B). CT angiography suggested a small irregularity of the right internal carotid artery (ICA). Diagnostic cerebral angiography confirmed a tiny, wide-necked blister aneurysm, 1.6 mm deepx2.9 mm wide at the neck, arising from the ophthalmic segment of the right ICA (Fig. 1C).
Given the aneurysm’s morphology and size, together with its ruptured status, flow diversion combined with placement of coils in the aneurysm was chosen as the best option for management. An external ventricular drain was placed for management of the hydrocephalus, and the patient was then loaded with aspirin and clopidogrel. A 6 Fr Benchmark catheter (Penumbra, Alameda, CA, USA) was placed in the right ICA. A Phenom 27 microcatheter (Medtronic, Irvine, CA, USA) was positioned into the right M1 middle cerebral artery segment in preparation for deployment of a Pipeline Embolization Device (Medtronic). Attempts were made to place a Headway Duo microcatheter (MicroVention Inc., Aliso Viejo, CA, USA) into the aneurysm but this proved extremely difficult. The stent was partially deployed, and this facilitated guidance of the coiling microcatheter into the aneurysm (Fig. 1D, E). At this point, the stent was covering the aneurysm neck, though was not fully deployed and detached. A 1.5 mmx4 cm MicroPlex 10 HyperSoft helical coil (MicroVention Inc.) was deployed into the aneurysm but detachment failed. Multiple repeated attempts at detachment were made, and the V-Grip detachment device was replaced in case it was faulty. The coil remained undetached.
At this point, we considered the position of the microcatheter, with its tip immediately adjacent to the flow diversion stent, meaning that the detachment zone was very likely in direct contact with the stent. We hypothesized that the high metal density in the flow diverter might be interfering with the electrothermal detachment of the coil. The coil was withdrawn a few millimeters into the microcatheter and detached immediately on the first attempt. The pusher wire was then advanced to push the tail of the coil into the aneurysm and successfully withdrawn. A second MicroPlex 10 HyperSoft coil (1.5 mmx3 cm) was deployed and detached in a similar fashion. The flow diverting stent was then fully deployed, and the coiling catheter was withdrawn (Fig. 1F).
Short-term follow-up with magnetic resonance angiography post-procedure demonstrated maintained stent patency and complete occlusion of the aneurysm. The patient provided written informed consent for publication of clinical information and related images.
Flow diversion stenting combined with placement of endovascular coils is a useful technique in various situations. It permits the treatment of very wide-necked aneurysms, blister aneurysms, or dysplastic vessels, while the addition of coils allows for immediate protection against rebleeding in ruptured aneurysms. Flow diverter-assisted coiling is particularly suited to the unique challenge posed by very small ruptured aneurysms [1]. The greater metal-to-artery ratio of flow diverters, as compared to conventional stents, provides better protection against migration of small-diameter coils. However, it appears that it may also interfere with electrothermal coil detachment. While coil detachment failure is a well-reported event, it did not appear to be an issue with a faulty coil or detachment device in this instance, given that replacing the device and pursuing numerous attempts at detachment were unsuccessful, until a small repositioning of the detachment zone was made away from the stent, and the coil was then detached immediately and uneventfully using the same device.
Failure of coil detachment is a potentially calamitous event. In the event that electrically mediated detachment fails, alternate methods have been described, including construction of a salvage connection (in the event of coil damage outside the rotating hemostatic valve) [2], mechanical detachment by rotation [3], and use of a 9-volt battery (MicroVention Inc., personal communication). These are not without risk, and rotation is the only method that might be expected to work in the situation of circuit disruption by stent material. Withdrawal of the coil is preferred if possible but may result in coil stretching. Coil stretching not only risks thromboembolic complications, but necessitates removal of the coiling microcatheter. If any further coil placement is needed, the aneurysm must be reaccessed. As this is not possible once a flow diverter has been deployed, the aneurysm could be left inadequately protected, or a second flow diverter could be required with attendant cost and potential complications.
An alternative explanation for the detachment failure could be compression or distortion of the detachment segment due to the high metal density of the stent strut. In this case, mechanically detachable coils might be susceptible to the same problem. It is also possible that heat dispersion away from the detachment zone was affecting the detachment mechanism. However, the solution would be the same in each case. This particular technical issue is most likely to arise in the setting of small, shallow aneurysms, where the tip of the jailed coiling catheter lies at the neck and immediately adjacent to the flow diverter. It may be circumvented by advancing the microcatheter tip away from the stent, if safe and feasible, or by positioning the detachment zone within the confines of the microcatheter. This technical issue and its solution have not been previously reported.
The optimal position of the detachment zone relative to the catheter tip has not been extensively studied, but is universally advised to be external to the catheter; indeed, Guglielmi’s initial clinical report described positioning the detachment point 3 mm beyond the catheter tip [4]. This was due to the change in electrical resistance in the system that occurs when the platinum-stainless steel junction zone is no longer insulated from the external environment by the plastic of the microcatheter [5]. Many of today’s coil systems do, however, permit detachment in the microcatheter, which may be employed beneficially in this setting. If detachment is undertaken in the microcatheter, following confirmation of successful detachment, it is crucial to advance the pusher wire to ensure the coil is fully out of the microcatheter before either placing another coil or withdrawing the microcatheter.
In summary, flow diverter-assisted coiling is useful in the management of very small, shallow, or blister aneurysms. Failure of coil detachment may result from contact between the flow diverter and the coil detachment zone. Withdrawing the coil a very short distance insulates the detachment zone within the microcatheter, and detachment can successfully occur. Recognizing the reason for detachment failure in this situation facilitates resolution and avoids potential disastrous complications.
Notes
REFERENCES
1. Dornbos D 3rd, Pillai P, Sauvageau E. Flow diverter assisted coil embolization of a very small ruptured ophthalmic artery aneurysm. J Neurointerv Surg. 2016; 8(e1):e2–e4.

2. Kim YS, Lee SW, Yeom JA, Yoon CH, Baik SK. Alternative detachment technique for electrically detachable coils: rescue from an unintended complication. Oper Neurosurg (Hagerstown). 2015; 11:569–574.

3. Lee CY, Yi MB, Benndorf G. Mechanical detachment of Guglielmi detachable coils after failed electrolytic detachment: rescue from a technical complication. Neurosurgery. 2008; 63(4 Suppl 2):293–294; discussion 294.

4. Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991; 75:8–14.
5. Guglielmi G. Accurate positioning of detachable coils using alternate electric current. A technical note. Interv Neuroradiol. 2006; 12:103–107.
Fig. 1.
(A) Diffuse subarachnoid hemorrhage with (B) evidence of diffuse intraventricular extension of hemorrhage and early hydrocephalus. (C) Initial cerebral angiogram demonstrating the wide-necked right internal carotid artery aneurysm, after which (D) the Pipeline Embolization Device (Medtronic, Irvine, CA, USA) is seen partially deployed before (E) placing the microcatheter tip within the aneurysmal sac. Final result (F) after deployment of 2 coils and full deployment of the Pipeline Embolization Device.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download