This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Three-dimensional (3D) measurement of intracranial aneurysms is important in planning endovascular treatment, and 3D rotational angiography (RA) is effective in accurate measurement. The purpose of this study was to evaluate the feasibility of low dose 3D RA (5 seconds 0.10 μGy/frame) in measuring an intracranial aneurysm using an in vitro phantom.
Materials and Methods
We investigated an in vitro 3D phantom of an intracranial aneurysm with 10 acquisitions of 3D RA with a conventional dose (5 seconds 0.36 μGy/frame) and 10 acquisitions with a low-dose (5 seconds 0.10 μGy/frame). 3D size and neck diameters of the aneurysm were measured and compared between the 2 groups (conventional and low-dose) using noninferiority statistics.
Results
The aneurysm measurements were well-correlated between the 2 readers, and noninferiority in the measurement of aneurysmal size of low-dose 3D RA was demonstrated, as the upper margin of the 1-sided 97.5% confidence interval did not cross the pre-defined noninferiority margin of 0.2 mm by the 2 readers.
Conclusion
Low-dose (5 seconds 0.10 μGy/frame) cerebral 3D RA is technically feasible and not inferior in in vitro 3D measurement of an intracranial aneurysm. Thus, low-dose 3D RA is promising and needs further evaluation for its clinical utility in the planning of endovascular treatment of an intracranial aneurysm.
Keywords: Cerebral angiography, Radiation dosage, Intracranial aneurysm
INTRODUCTION
Three-dimensional (3D) rotational angiography (RA) has a pivotal role in the evaluation of intracranial aneurysms, including accurate assessment of size as well as detection and pretreatment evaluation of aneurysms [
1-
6]. Although there have been reports showing the feasibility of low-dose 3D RA, little work has been done to study the effectiveness of accurate measurement of an aneurysm using low-dose 3D RA [
7,
8].
In this brief report, we aimed to evaluate the feasibility of the lowest possible dose of 3D RA (5 seconds 0.10 μGy/frame) by our machine to measure an intracranial aneurysm. We investigated an in vitro phantom of an intracranial aneurysm, and the measurements were compared with those from 3D RA with a conventional dose (5 seconds 0.36 μGy/frame).
MATERIALS AND METHODS
The 3D replica of an intracranial aneurysm was designed by a vascular neurosurgeon (JHC) and printed with a 3D printer (Projet MJP 2500 Plus 3D; 3D Systems, Rock Hill, SC, USA) using an elastic polymer rubber material. It was originally printed for education and hands-on purposes for endovascular treatment of a large aneurysm with a complex vasculature, and the detailed description of making this 3D replica will be described separately. For the in vitro phantom study, a 3D replica was filled with iodixanol 270 mg/mL (Visipaque 270; GE Healthcare, Amersham, England) and placed in a saline chamber. Using a biplane flat detector angiography unit (Axiom Artis Zee; Siemens, Erlangen, Germany), a total of 20 acquisitions of 3D RA were performed; 10 acquisitions with a conventional dose (5 seconds 0.36 μGy/frame) and 10 acquisitions with a low-dose (5 seconds 0.10 μGy/frame). The common parameters were as follows: 5-second rotation; rotation angle, 200 degrees with 1.5-degree increments resulting in 133 projections; rotation speed, 40 degree/s; acquisition matrix, 1,240×960. Acquired data were sent to the workstation (Syngo 3D workplace; Siemens AG, Erlangen, Germany) and 3D reconstruction was performed with the following parameters; kernel type, Hounsfield unit; image characteristics, normal; reconstruction mode, native; slice matrix, 512×512, and voxel size, 0.47 mm.
All the volume rendering (VR) images were displayed using factory-default settings for the visualization of an intracranial aneurysm, in an attempt for consistency of the datasets measured. Twenty sets of VR reconstruction images were sorted in random order and investigated in blinded evaluation separately by 2 experienced neurointerventionists (BSK, HJK). Height, width, depth, and neck size of the aneurysm were measured on a dedicated workstation.
For the statistical analysis for the phantom study, noninferiority tests were used for the comparison of measured parameters of the aneurysm between group 1 with conventional 3D RA (0.36 μGy/frame) and group 2 with low-dose 3D RA (0.10 μGy/frame). Because there has been no general use of predefined noninferiority margin of size measurement of an aneurysm, we set 0.2 mm as an acceptable noninferiority margin. Interrater reliability was tested using the Cronbach α.
RESULTS
The measured values of VR reconstructed images of the 3D replica by 2 neurointerventionists are summarized in
Table 1. The mean size of the aneurysm neck (±standard deviation) was 8.23±0.08 mm in group 1, 8.20±0.07 mm in group 2 by reader 1, and 8.11±0.04 mm, 8.06±0.05 mm by reader 2, respectively. The mean height was 11.33±0.04 mm in group 1, 11.31±0.09 mm in group 2 by reader 1, and 10.99±0.13 mm, 10.94±0.08 mm by reader 2, respectively. The mean width was 12.01±0.03 mm in group 1, 11.92±0.08 mm in group 2 by reader 1, and 11.94±0.07 mm, 11.90±0.05 mm by reader 2, respectively. The mean depth was 14.42±0.06 mm in group 1, 14.40±0.07 mm in group 2 by reader 1, and 14.76±0.05 mm, 14.68±0.04 mm by reader 2, respectively.
The measured aneurysm sizes were well-correlated between the 2 readers (group 1, Cronbach’s α=0.997; group 2, Cronbach’s α=0.997). Moreover, the noninferiority in measurements (aneurysm neck, height, width, and depth) of lowdose 3D RA (0.10 μGy/frame) was demonstrated, since the upper margin of the 1-sided 97.5% confidence interval did not cross the predefined noninferiority margin of 0.2 mm (
Fig. 1).
DISCUSSION
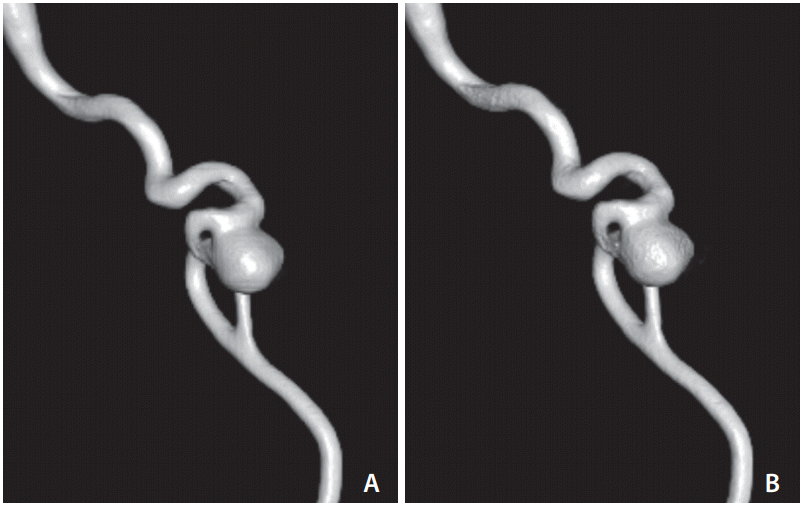
In measuring the size of the aneurysm in our
in vitro phantom study, the measured values in group 1 (5 seconds 0.36 μGy/frame;
Fig. 1A) tended to be larger than those of group 2 (5 seconds 0.10 μGy/frame;
Fig. 1B), but the margin of the 1-sided 97.5% confidence interval did not cross the predefined noninferiority margin of 0.2 mm. Therefore, we showed that low-dose 3D RA was not inferior in terms of measuring aneurysm size. Pearl et al. [
8] evaluated 3D RA of varying acquisition time and dose in 3 swines and compared mean differences in arterial diameter measurements between protocols. Their results showed that measurement differences between the standard and low dose protocols were not clinically significant (<0.5 mm). Although it was the measurement of arterial diameter, their results were similar to our results with an intracranial aneurysm. For the measurement of an intracranial aneurysm, Pearl et al. [
7] also evaluated the difference in measurement between conventional and various low dose 3D-RA protocols. In their reports, there were differences of more than 0.5 mm between the 5 seconds 0.36 μGy/frame and the 5 seconds 0.10 μGy/frame. However, the comparison between these 2 protocols was just the range of difference from a single measurement of only 4 subjects and was not validated as a statistical comparison of measurements. On the contrary, this study had repeated acquisitions of 3D RA of an aneurysm phantom, and analysis was done with noninferiority statistics to show low-dose 3D RA using 0.10 μGy/frame was not inferior in terms of 3D measurement of aneurysm size by the margin of 0.2 mm, which seemed to be clinically acceptable.
Theoretically, by changing the 3D RA protocol from group 1 (0.36 μGy/frame) to group 2 (0.10 μGy/frame), we may expect about a 72% of reduction of radiation dose from the acquisition of each 3D RA. In the report of Choi et al. [
9], the proportion of dose-area-product of 3D RA over total patient radiation dose ranged from 18% to 36%. Therefore, by clinical application of a new protocol with low-dose 3D RA in the evaluation of an intracranial aneurysm, we may estimate a 13–26% reduction in total patient radiation dose. However, it should be validated in a patient cohort; furthermore, the evaluation of subjective image quality, including the visibility of small branches or perforating vessels, should be further evaluated by an in vivo study.
There are some limitations in this brief report. First, the aneurysm phantom was located in a saline chamber during the acquisition of 3D RA, thus attenuation of X-ray by the skull could not be considered in this in vitro study. Second, pulsatile flow was not applied to the aneurysm phantom. Pulsatile movement of the vascular wall averaged during 5 seconds of 3D RA acquisition could create artifacts, which could be more prominent with low-dose radiation. But, it was evaluated in this study with a relatively rigid phantom without pulsatile flow. Third, although repeated measurements of 3D RA were performed, only a single replica of an intracranial aneurysm phantom was used. We assume that the in-vivo evaluation of a larger number of aneurysms in the patient cohort may overcome these limitations.
CONCLUSION
In conclusion, low-dose (5 seconds 0.10 μGy/frame) cerebral 3D RA was technically feasible and not inferior in the in vitro 3D measurement of an intracranial aneurysm, with a considerable reduction in radiation dose. Thus, low-dose 3D RA is promising and needs further evaluation for its clinical utility in the planning of endovascular treatment of an intracranial aneurysm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download