Efforts to reduce radiation dose during neurointervention may be underappreciated because brief exposure to radiation seems harmless; however, radiation during neurointervention induces stochastic effects such as gene expression alterations and deterministic effects such as cataract formation [1,2]. For these reasons, neurointerventionists should understand and implement the principle of “as low as reasonably achievable (ALARA)” every time they step on the fluoroscopy pedal. The following article is a summary of recent strategies in neurointervention that may allow for reducing radiation dose.
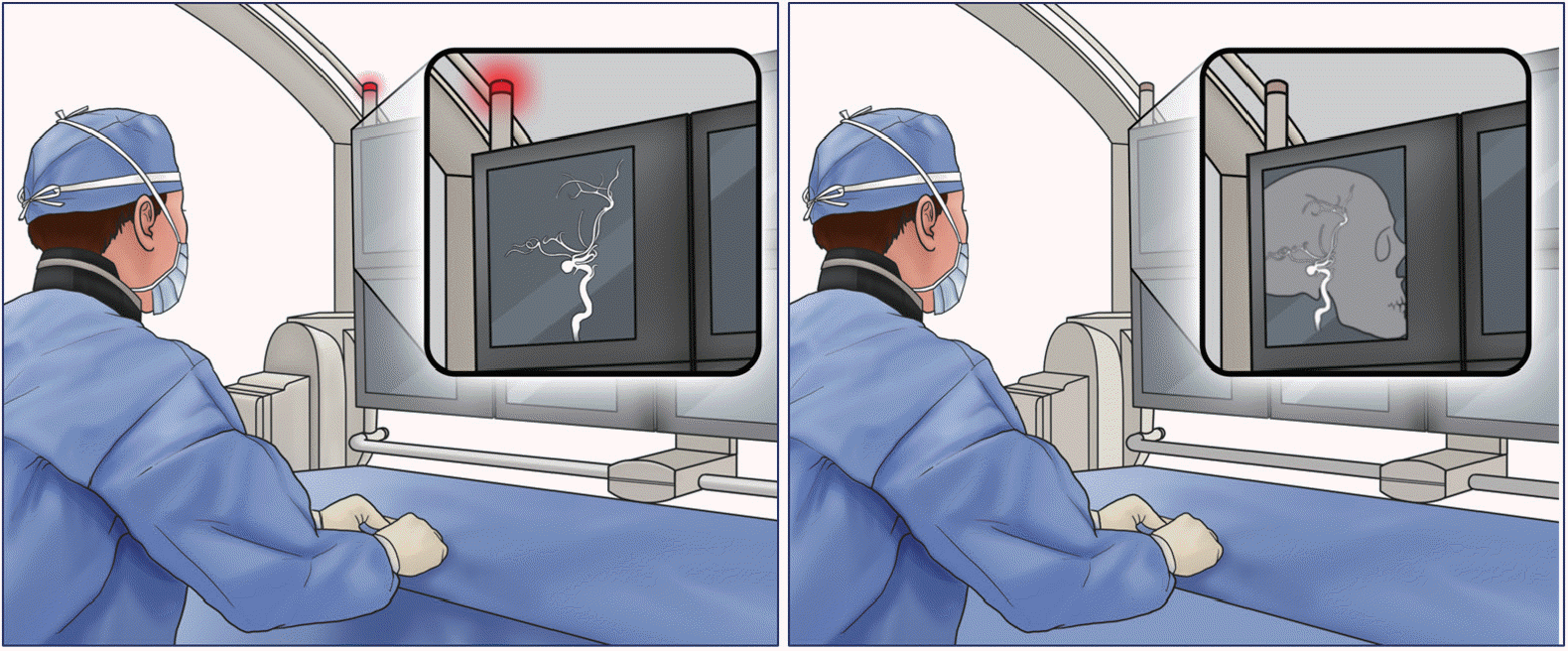
The optimization of fluoroscopy settings such as frame rate, filter options, automatic exposure control, focal spot, current, and peak potential according to each specific procedure is critical (Fig. 1). Not every vascular analysis warrants 2D or 3D digital subtraction angiography. Likewise, not every fluoroscopy projection warrants the same pulse rate, focal spot, and frame rates. Customized low-dose protocols can be created by calibrating the above-mentioned fluoroscopy settings. For instance, by lowering the fluoroscopy pulse rate from 15 to 7.5 pulses per second, a dose reduction of 30% was achieved in the fluoroscopy mode and of 47% in the digital subtraction angiography mode [3]. Recent studies have demonstrated that the application of low-dose protocols is effective and safe for diagnostic angiography. Song et al. [4] created a low-dose protocol by gradually decreasing the detector entrance dose from 23 to 8 nGy, which lowered the fluoroscopy dose area product (DAP) and air kerma (AK) by 52% in diagnostic cerebral angiography, as compared to default settings. Also, van der Marel et al. [5] created a dose-reduction platform, and its application reduced cumulative DAP in both diagnostic and neurointervention procedures by 53.2%. Kim et al. [6] demonstrated that the addition of filters can greatly decrease radiation dose in vitro and in vivo. Reductions in AK (up to 40–50% per patient) and DAP (up to 25–40% per patient) were achieved during diagnostic angiography. To optimize fluoroscopy settings and protocols, a meticulous review of every procedure and evaluation of dose reports are necessary. Additionally, it is essential to consider the expertise and advice of technicians and physicists who specialize in this field.
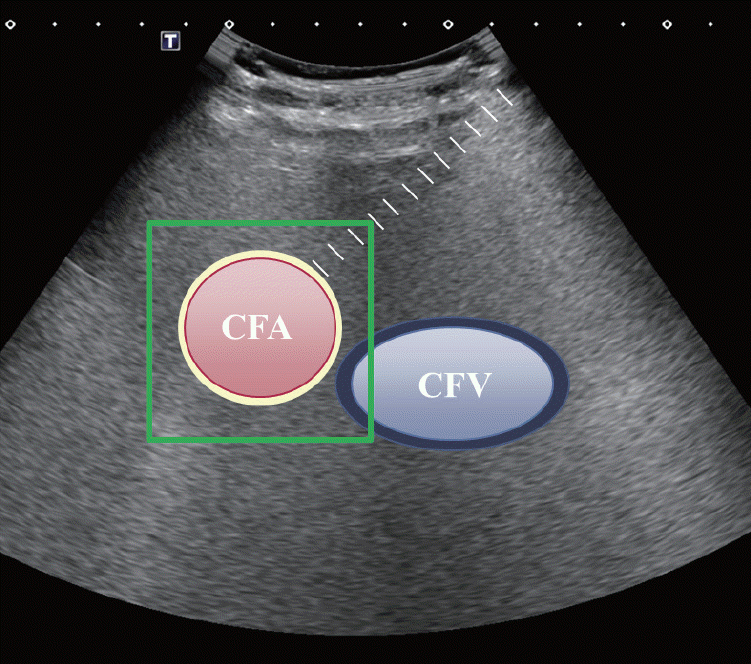
Ultrasonography is considered an adjunct modality for arterial or venous access to avoid unnecessary fluoroscopy projection and wire navigation (Fig. 2). Whether it is used to perform a direct puncture into a venous malformation or to gain femoral artery or venous access, ultrasonography can be an invaluable tool to achieve an accurate puncture and localization without radiation. Slattery et al. [7] demonstrated that by using ultrasonography for common femoral artery access, 1 minute 55 seconds of procedure time and mean DAP 199 cGy cm2 of radiation dose can be reduced, compared to a fluoroscopy-guided approach. Also, Stone et al. [8] demonstrated that ultrasound-guided femoral puncture had a faster median cannulation time (80 seconds vs. 100 seconds) and a higher success rate (93% vs. 86%) compared to fluoroscopy-guided femoral puncture in a prospective randomized control study with 635 patients. Therefore, the application of ultrasonography should be considered in order to reduce radiation exposure, procedure time, and complication risk.
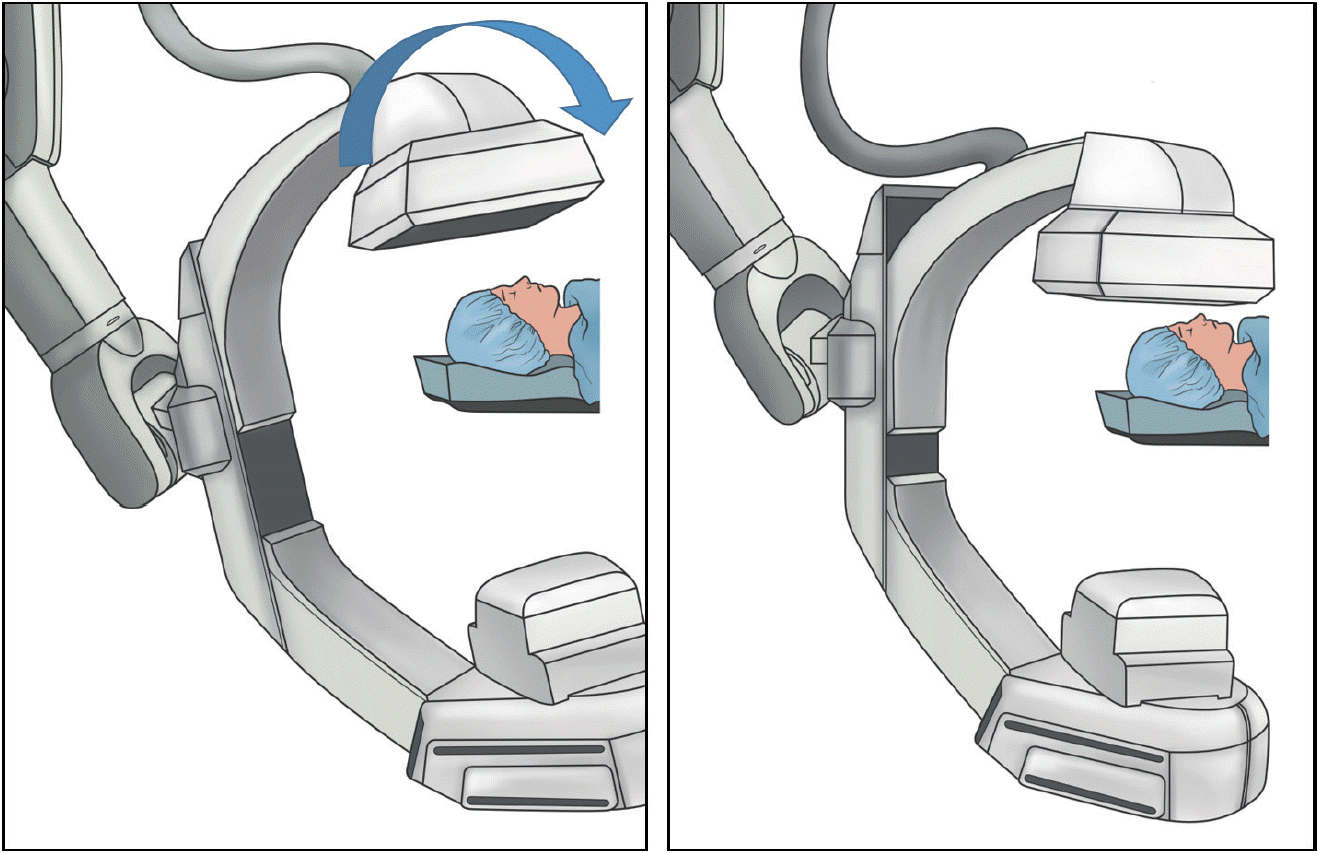
Smaller craniocaudal angulation or true posteroanterior (PA) projection may result in less radiation exposure than the conventional projection (Fig. 3). In a phantom study, Song et al. [9] demonstrated that an increase in the cranial angulation or caudal angulation resulted in a greater radiation dose during routine cerebral angiography. Specifically, a cranial angulation of 20 degrees increased the AK by 5.4%, compared to the true PA projection. Even with copper filter applications, a similar tendency was observed, except for applications with a 0.2-mm copper filter. In an in vivo study of 31 patients, a mean dose reduction of 11% was achieved by the application of PA projection during diagnostic angiography. Although the expected overlap between the skull base and vessels was much higher in the PA projection than in the conventional projection, the visibility of cerebral aneurysms at various locations, using a 4-point scale, was statistically insignificant between two projections.
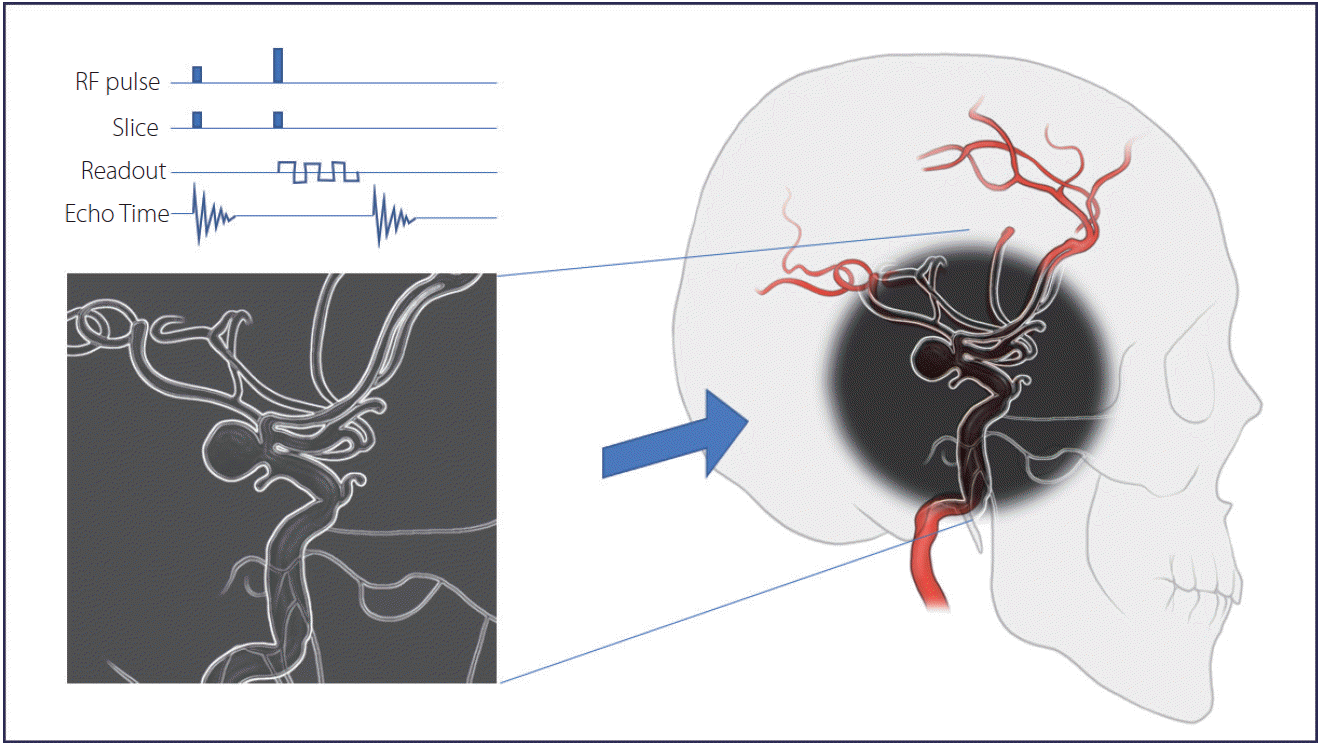
Fusion of 3D magnetic resonance angiography (MRA) with 2D digital subtraction angiography may also reduce the radiation dose. Modern fluoroscopy can import and overlay images from high-resolution MRA and use them as a real-time “virtual” roadmap (Fig. 4). Overlay mapping via 3D MRA may provide various advantages to neurointerventionists in terms of enhanced spatial resolution and capture of surrounding anatomy during the procedure, which ultimately may reduce the procedure time, contrast media injection, and overall radiation dose. In a retrospective study, Jang et al. [10] demonstrated that the fusion of 3D MRA with 2D monoplane is feasible and safe for coil embolization of both unruptured and ruptured cerebral aneurysms. From February 2013 to July 2013, coil embolization of 33 patients with this fusion technique had a lower mean radiation dose (3,375 mGy vs. 1,953 mGy), lower fluoroscopy time (62.3 minutes vs. 30.5 minutes), and shortened procedure time (105 minutes vs. 82.2 minutes) in comparison to that of 27 patients who were evaluated using conventional methods. There were no differences in procedural or perioperative complications between the 2 groups (4 vs. 2 patients and 4 vs. 3 patients, respectively).
In conclusion, there are numerous ongoing studies that reflect neurointerventionists’ endeavors and strategies to reduce radiation dose during procedures. Whether performing a simple diagnostic angiography or complex coil embolization for a cerebral aneurysm, a neurointerventionist should always remember and implement the principle of “ALARA”.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download