This article has been corrected. See "Title Correction: Transcarotid Mechanical Thrombectomy for Embolic Intracranial Large Vessel Occlusion after Endovascular Deconstructive Embolization for Carotid Blowout Syndrome" in Volume 15 on page 171.
Abstract
Carotid blowout syndrome (CBS) is a fatal complication of head and neck cancer. Endovascular treatment, particularly deconstructive embolization, is effective for CBS, but it might result in thromboembolic events. We report the case of a 57-year-old man with underlying recurrent head and neck cancer who had CBS. The patient received endovascular embolization of the right internal, external, and common carotid arteries. Right internal carotid artery to middle cerebral artery embolic occlusion was noted immediately after the procedure, and left-sided weakness and facial palsy were found. Ipsilateral suprabulbar cervical internal carotid artery puncture was performed under fluoroscopic guidance, and rescue suction thrombectomy was successful. The patient had no significant neurological sequela. Transcarotid intraarterial thrombectomy is a reasonable method for managing postembolization large vessel occlusion, even in the neck, after irradiation.
Carotid blowout syndrome (CBS) is a fatal complication of head and neck cancer. As survival after successful treatment of CBS can be 6–12 months or longer [1,2], early recognition and treatment of CBS may provide a favorable outcome for these patients. Endovascular treatment has been known to be effective for CBS, which can cause life-threatening situations with contrast leakage or pseudoaneurysms [3]. Prompt treatment is warranted once diagnosed. However, ischemic stroke, resulting from either hemodynamic insufficiency or a thromboembolic event, is a major concern with endovascular treatment. Thromboembolism can be caused by an embolic agent or acute thrombus, occasionally leading to intracranial large vessel occlusion and debilitating stroke. The treatment of the aforementioned complication is difficult because contraindications of current standard revascularization therapies may be encountered.
Here, we present a case of a massive right internal carotid artery (ICA)-middle cerebral artery (MCA) clot after endovascular carotid trunk embolization. We treated this complication successfully with endovascular suction thrombectomy via direct ICA puncture.
Data were obtained from the stroke registry at the National Taiwan University Hospital (NTUH), which was established in 1995 to study the etiologic factors, clinical courses, prognoses, and complications of stroke. The study was approved by the Research Ethics Committee of the hospital. Individual informed consent was waived.
A 57-year-old man presented to our emergency department complaining of massive nasal and oral bleeding over the previous day. The patient had a history of head and neck squamous cell carcinoma (right buccal cancer and oropharyngeal cancer, diagnosed 9 and 7 months earlier, respectively), for which he had undergone wide excision of the tumor, neck dissection, and tracheostomy and had been receiving concurrent chemoradiotherapy until 5 months before the current presentation. On examination, a mild fever (body temperature, 38.3°C) and tachycardia (125 beats/min) were noted. He was completely conscious. Laboratory investigation showed low levels of hemoglobin (8.3 g/dL) and hyponatremia (128 mmol/L). No other major physical or blood abnormalities were noted. The otolaryngologist found no airway compromise or active bleeding, but noted significant blood clots in the nasopharynx and oropharynx that could not be totally removed. Intravenous tranexamic acid, blood component therapy, and epinephrine gauze packing in the oral cavity were administered. Computed tomographic angiography (CTA) was performed to detect the suspected vascular lesion. The result revealed a 4-mm pseudoaneurysm, starting from the right inferior thyroid artery. It also showed a right carotid bulb was encased by a necrotic tumor (Fig. 1A, B). These findings suggested tumor recurrence and impending CBS. CTA at the circle of Willis (COW) level revealed a robust anterior communicating artery, proximal bilateral anterior cerebral arteries, and a right posterior communicating artery (Fig. 1C-E, respectively). The neurointerventional team was consulted and decided to perform embolization of the right inferior thyroid artery and right carotid artery.
The patient was sent to the neuroangiography suite 13 hours after this visit. First, right inferior thyroid artery pseudoaneurysm embolization was performed with pushable coils (Fig. 2A, B). Subsequently, right carotid angiography confirmed ulceration and luminal irregularity at the carotid bulb, compatible with vascular encasement and impending blowout (Fig. 2C). Deconstructive endovascular carotid trunk embolization was then performed, starting from the external carotid artery and the proximal cervical segment of the ICA by using pushable coils (Nester embolization coil; Cook Medical, Bloomington, IN, USA), followed by a vascular plug (AMPLATZER™ Vascular Plugs; St. Jude Medical, St. Paul, MN, USA) placement to the common carotid artery (Fig. 2D). After the embolization, contralateral carotid and vertebral angiography were performed to check for cerebral perfusion. Poor collateral flow to the right terminal ICA was noted, along with a filling defect extending from the ICA to the proximal MCA (Fig. 2E, F). Acute thrombus formation with intracranial migration was diagnosed. Immediate neurological examination revealed left facial palsy and mild left side weakness. The patient’s National Institutes of Health Stroke Scale (NIHSS) score was 6. After discussion with consulting neurologists, an intravenous thrombolytic agent was not administered because of the ongoing hemorrhage. Endovascular mechanical thrombectomy via ipsilateral carotid puncture was thus planned.
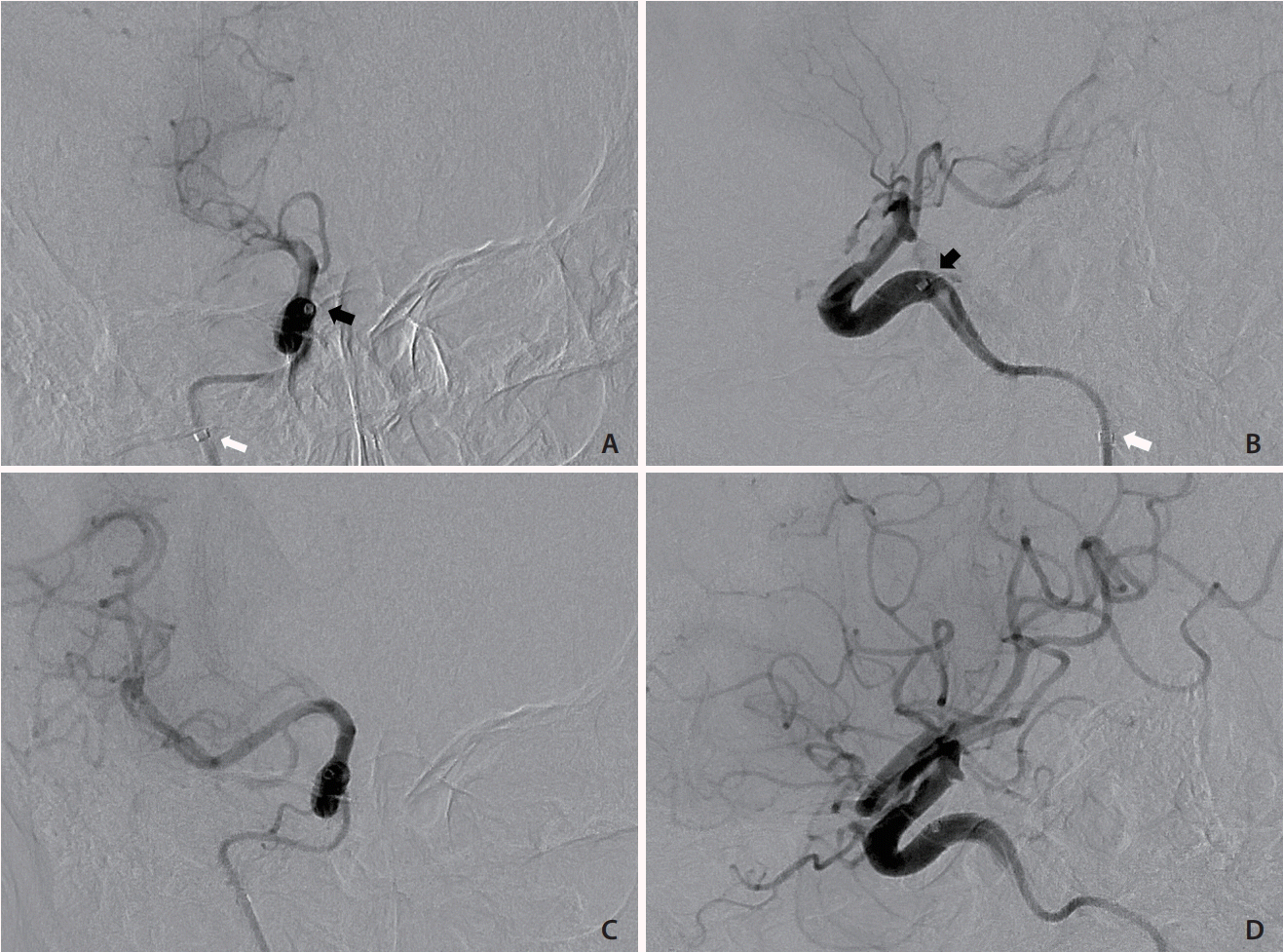
With the patient under local anesthesia, the procedure was started 90 minutes after the onset of weakness. Sonographic guidance was attempted but was unsuccessful because of neck rigidity and a poor sonographic window. After prior embolization, the radiopaque coils in the right carotid artery were precise landmarks under fluoroscopy. The right carotid angiography before embolization was also used as a guidance of the right ICA. A 21-gauge needle set (percutaneous transhepatic needle cholangiography [PTC]; Hakko Co., Tokyo, Japan) was inserted into the middle cervical portion of the right ICA under fluoroscopic guidance (Fig. 3A-D). A 0.014-inch microwire was then inserted via the needle to check whether the needle tip was in the right ICA or not. The microwire coursed smoothly along the right ICA without resistance, loop, or kink, which indicated successful ICA puncture. Track dilation was difficult because of the extreme stiffness of the irradiated neck. Sequential dilation of the tract was performed using an 18-gauge PTC needle and vascular sheaths (5 and 6 French; Medikit Co., Tokyo, Japan), and a 5-French guiding sheath (Flexor Shuttle Guiding Sheath; Cook Medical, Bloomington, IN, USA) was finally placed in the upper cervical ICA (Fig. 3E). Repeated right ICA angiography revealed total occlusion of the right MCA (Fig. 4A, B). Systemic heparin was not given because of the ongoing hemorrhage. Suction thrombectomy was performed using a 5-French intermediate catheter (Navien 058; Covidien Vascular Therapies, Mansfield, MA, USA), and Thrombolysis in Cerebral Infarction grade 3 reperfusion was achieved in the first pass (Fig. 4C, D). Embolization of right high cervical ICA with pushable coils was then performed to prevent retrograde blood flow. After removal of the sheath, hemostasis was achieved by manual compression of the tract for 5 minutes. The total procedure time was 90 minutes. The patient’s neurological status improved during the following day with an NIHSS score of 1 point. No major bleeding episode occurred during admission. The patient was successfully discharged 12 days after presentation.
At 73 days after the last discharge, the patient was admitted again because of poor oral intake. Oral bleeding occurred during hospitalization. The CTA examination revealed a pseudoaneurysm located in the tongue base. Complete obliteration of the right carotid trunk was confirmed, but the recurrent tumor had progressed. Another session of endovascular embolization via the left lingual artery was performed successfully. The patient was followed up in the clinic thereafter without neurological deficit.
During neurointerventional procedures, acute intracranial large vessel occlusion is a condition prompting immediate revascularization if possible. Pushable coils are more thrombogenic than detachable coils during embolization. The thrombus after proximal ICA occlusion could originate from stasis of the blood flow in ICA with distal migration by collateral flow into the petrocavernous ICA, or from the thrombus formation in common carotid artery (CCA) or external carotid artery (ECA) through ECA-ICA anastomosis. A thrombus beyond the proximal ICA occlusion was the main issue in our patient because of the large thrombus burden [4]. Our patient had a relative contraindication for intravenous thrombolytic therapy because of active hemorrhage. Surgical embolectomy was a possible choice, but this was limited by the lack of an experienced neurosurgeon at the hospital. Endovascular mechanical thrombectomy was the preferred choice, but the standard femoral approach was not feasible because the carotid trunk had been occluded. We therefore established vascular access via ICA puncture. The procedure must be performed under fluoroscopic guidance because of the rigidity of the irradiated neck.
Treatment of CBS is critical for survival [1]. Currently, most patients with CBS are treated via an endovascular approach. Surgical treatment results in more neurological complications than endovascular treatment. In endovascular treatment, a covered stent is used for reconstructive procedures [5]. Nevertheless, covered stent placement results in the likelihood of recurrent bleeding, delayed thromboembolic events, and infectious complications. It therefore has a longterm outcome worse than a deconstructive procedure [6]. Evaluation of the COW could predict the collateral condition of the patient undergoing carotid embolization [7]. Therefore, a covered stent should be reserved for a bridging procedure indicated for patients with a poor COW or intolerance to an occlusion test [8]. In our patient, the COW integrity was robust; therefore, we selected deconstructive embolization as the treatment procedure. During follow-up, despite tumor progression and right carotid space invasion, no life-threatening hemorrhage from the right carotid trunk occurred. The neurological condition of the patient returned to nearly normal after successful salvage thrombectomy, supporting the preprocedural assessment.
In 1927, Egas Moniz pioneered diagnostic cerebral angiography by surgical cutdown of the ICA. In the subsequent years, a direct percutaneous approach to the ICA followed by femorocerebral angiography was developed [9]. Currently, direct carotid artery access is used as an alternative to the transfemoral approach for endovascular procedures when the tortuosity and stenosis cannot be overcome. Numerous types of endovascular interventions can be administered via carotid artery access, including embolization, angioplasty, stenting, and thrombectomy, even in children [10-12]. CCA is a common access site with the direct carotid approach. By contrast, the ICA approach is used for treating CCA or proximal ICA diseases, with the cervical segment of the ICA being used most frequently. In our patient, because ultrasonographic identification of the upper ICA was difficult, preprocedural angiography and a deployed coil were used for fluoroscopic guidance.
Endovascular mechanical thrombectomy is an important contemporary stroke management procedure. Similar to other neurointerventional procedures, transcarotid access is feasible for performing thrombectomy. This technique may be included in the arsenal of the stroke interventionist because there is a high probability of encountering excessive tortuosity in elderly patients [10,13]. Current thrombectomy devices typically require relatively large bore-guiding catheters to accommodate them. Local complications, including local hematoma and vascular dissection, are a concern after vascular sheath removal. Elective intubation for airway protection has therefore been advocated [10,13,14]. Manual compression 10–30 minutes is typically adequate for hemostasis. By contrast, vascular closure devices, such as Angio-Seal (Terumo, Tokyo, Japan), Mynx (Cardinal Health, Dublin, OH, USA), and Perclose ProGlide (Abbott Vascular, Santa Clara, CA, USA), have been used in extracranial carotid arteries [10,13-16]. In our patient, coil embolization of the high cervical ICA distal to the entry point was performed, leaving the puncture site isolated from the blood supply.
Notes
REFERENCES
1. Lu HJ, Chen KW, Chen MH, Chu PY, Tai SK, Wang LW, et al. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J Vasc Surg. 2013; 58:1226–1235.

2. Lee CW, Yang CY, Chen YF, Huang A, Wang YH, Liu HM. Ct angiography findings in carotid blowout syndrome and its role as a predictor of 1-year survival. AJNR Am J Neuroradiol. 2014; 35:562–567.

3. Zhao LB, Shi HB, Park S, Lee DG, Shim JH, Lee DH, et al. Acute bleeding in the head and neck: angiographic findings and endovascular management. AJNR Am J Neuroradiol. 2014; 35:360–366.

4. Capo H, Kupersmith MJ, Berenstein A, Choi IS, Diamond GA. The clinical importance of the inferolateral trunk of the internal carotid artery. Neurosurgery. 1991; 28:733–737. ; discussion 737-738.

5. Gaynor BG, Haussen DC, Ambekar S, Peterson EC, Yavagal DR, Elhammady MS. Covered stents for the prevention and treatment of carotid blowout syndrome. Neurosurgery. 2015; 77:164–167.

6. Chang FC, Lirng JF, Luo CB, Guo WY, Teng MM, Tai TS-K, et al. Carotid blowout syndrome in patients with headandneck cancers reconstructive management by selfexpandable stent-grafts. AJNR Am J Neuroradiol. 2007; 28:181–188.
7. Lee BC, Lin YH, Lee CW, Liu HM, Huang A. Prediction of borderzone infarction by CTA in patients undergoing carotid embolization for carotid blowout. AJNR Am J Neuroradiol. 2018; 39:1280–1285.

8. Suárez C, Fernández-Alvarez V, Hamoir M, Mendenhall WM, Strojan P, Quer M, et al. Carotid blowout syndrome: modern trends in management. Cancer Manag Res. 2018; 10:5617–5628.

9. Artico M, Spoletini M, Fumagalli L, Biagioni F, Ryskalin L, Fornai F, et al. Egas moniz: 90 years (1927-2017) from cerebral angiography. Front Neuroanat. 2017; 11:81.

10. Roche A, Griffin E, Looby S, Brennan P, O’Hare A, Thornton J, et al. Direct carotid puncture for endovascular thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019; 11:647–652.

11. Sfyroeras GS, Moulakakis KG, Markatis F, Antonopoulos CN, Antoniou GA, Kakisis JD, et al. Results of carotid artery stenting with transcervical access. J Vasc Surg. 2013; 58:1402–1407.

12. Choudhry S, Balzer D, Murphy J, Nicolas R, Shahanavaz S. Percutaneous carotid artery access in infants < 3 months of age. Catheter Cardiovasc Inter. 2016; 87:757–761.
13. Mokin M, Snyder KV, Levy EI, Hopkins LN, Siddiqui AH. Direct carotid artery puncture access for endovascular treatment of acute ischemic stroke: technical aspects, advantages, and limitations. J Neurointerv Surg. 2015; 7:108–113.

14. Jadhav AP, Ribo M, Grandhi R, Linares G, Aghaebrahim A, Jovin TG, et al. Transcervical access in acute ischemic stroke. J Neurointerv Surg. 2014; 6:652–657.

Fig. 1.
Preprocedural CTA. (A) CTA coronal view showing a 4-mm pseudoaneurysm (arrow) of the right inferior thyroid artery. (B) CTA sagittal view showing the encased right carotid bulb and proximal ICA with focal luminal narrowing (arrow). (C) Axial view of COW showing robust Acom (arrow) and proximal bilateral ACAs (arrowheads). (D, E) Axial and sagittal views of COW showing robust right Pcom (arrows). CTA, computed tomographic angiography; ICA, internal carotid artery; COW, circle of Willis; Acom, anterior communicating artery; ACAs, anterior cerebral arteries; Pcom, posterior communicating artery.
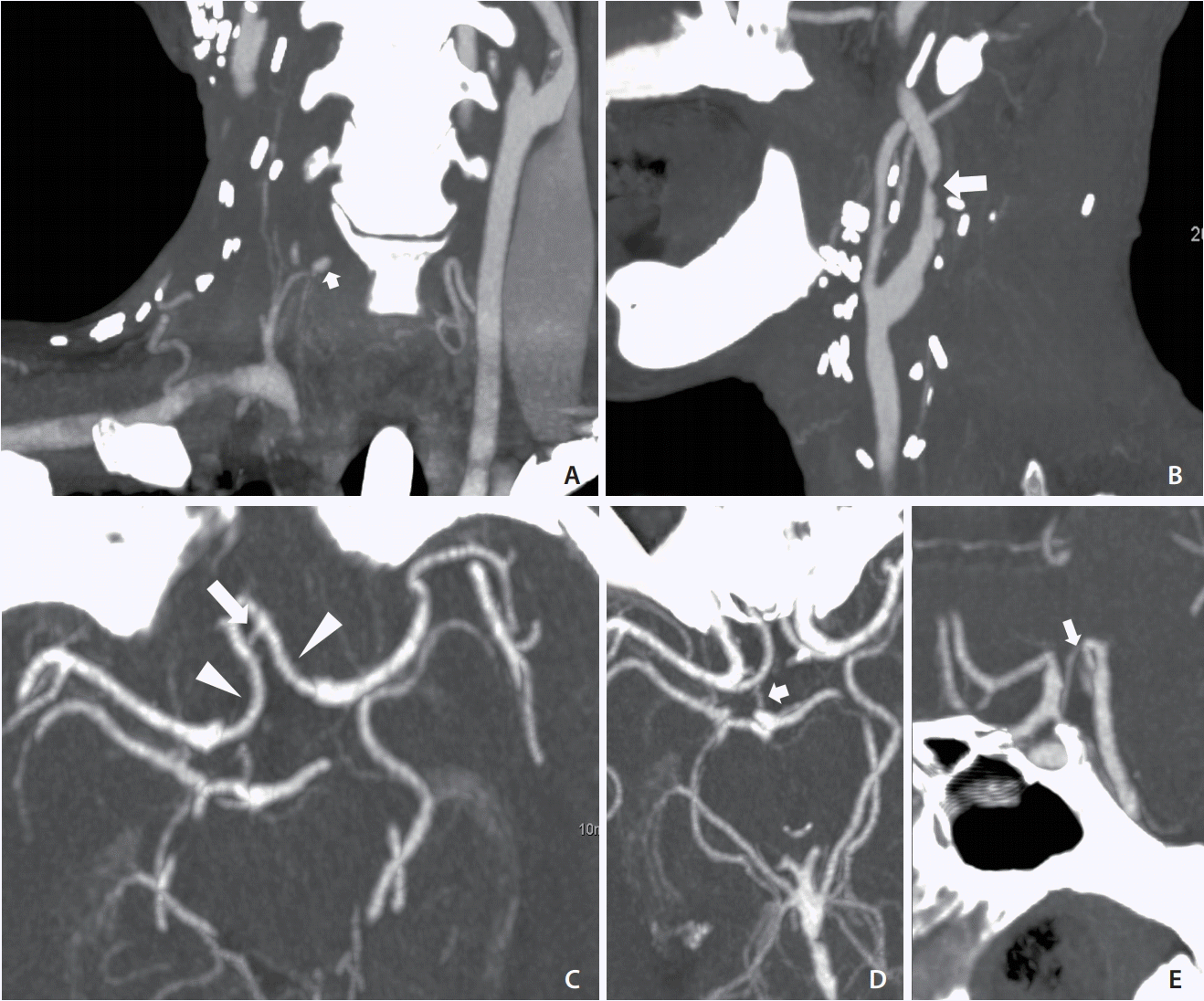
Fig. 2.
Endovascular embolization of pseudoaneurysm of the right inferior thyroid artery and right carotid artery. (A) Frontal view of right subclavian artery angiography demonstrating a small pseudoaneurysm (arrow). (B) Postembolization of right inferior thyroid artery showing deployed coils and obliteration of the pseudoaneurysm. (C) Right CCA lateral angiography showing the ulceration (arrowhead) and focal narrowing (arrow) at the carotid bulb, compatible with vascular encasement and impending blowout. (D) Frontal fluoroscopic spot view after endovascular carotid trunk embolization revealing radiopaque coils in the ECA (arrow) and ICA (arrowhead) as well as a vascular plug in the CCA (black arrow). (E, F) Frontal and lateral views of left vertebral angiography showing a filling defect from the right ICA (arrows) to the proximal MCA (arrowheads). CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery.
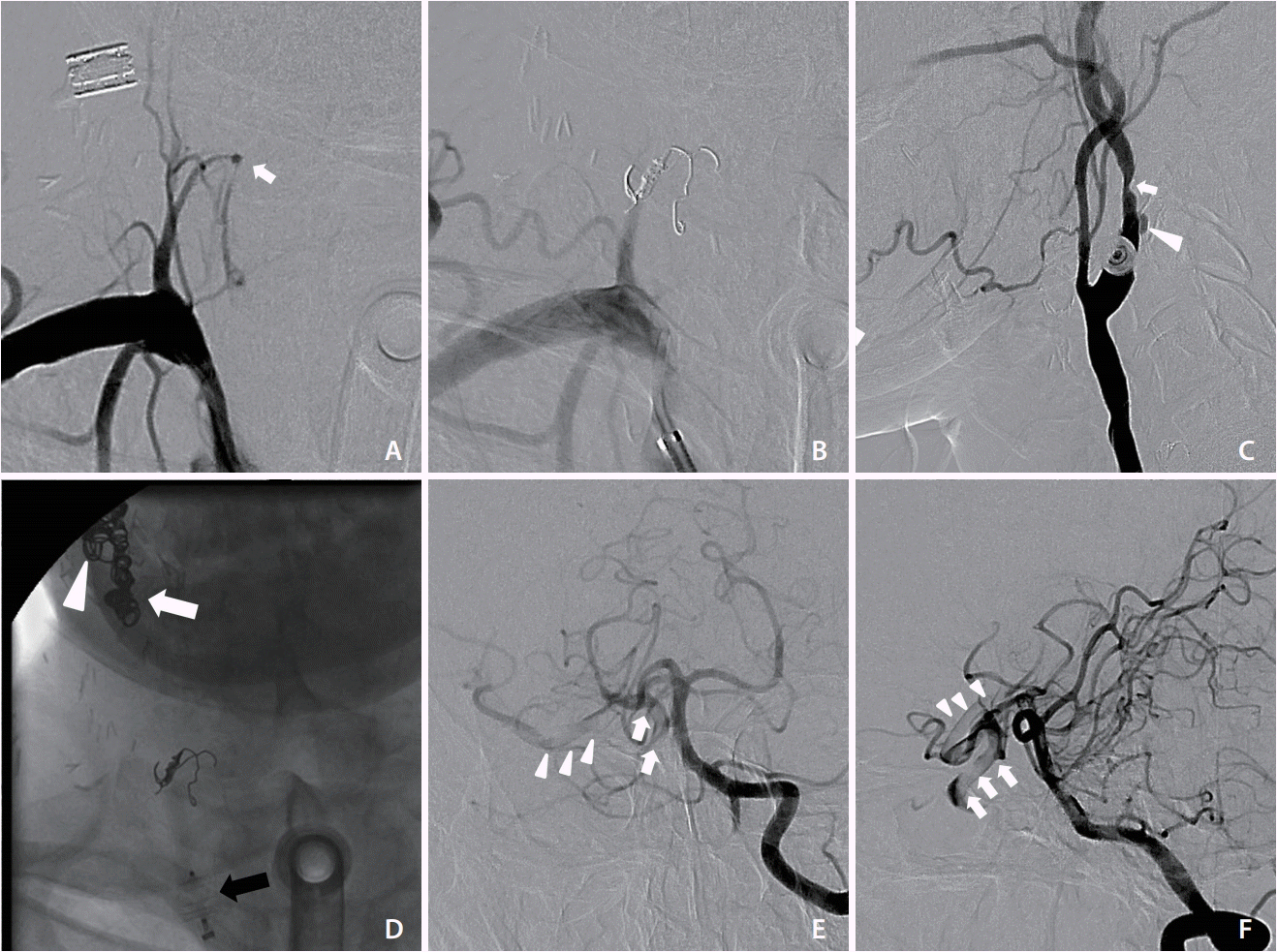
Fig. 3.
Fluoroscopically guided direct puncture of the right ICA. (A, B) Frontal and lateral views of fluoroscopic spot views showing an imaginary direction of the ICA (dashed lines), using deployed coils as precise landmarks. The puncture needle (asterisks) was inserted toward the imaginary line. (C, D) Frontal and lateral views of right carotid angiography before embolization showed the course of the right ICA (dashed lines), which gave the imaginary guidance of ICA puncture. (E) The right neck puncture site showing the 6-French vascular sheath (white arrow), whose tip was just outside the carotid sheath, and the 5-French guiding sheath (black arrow) into the right ICA. ICA, internal carotid artery.
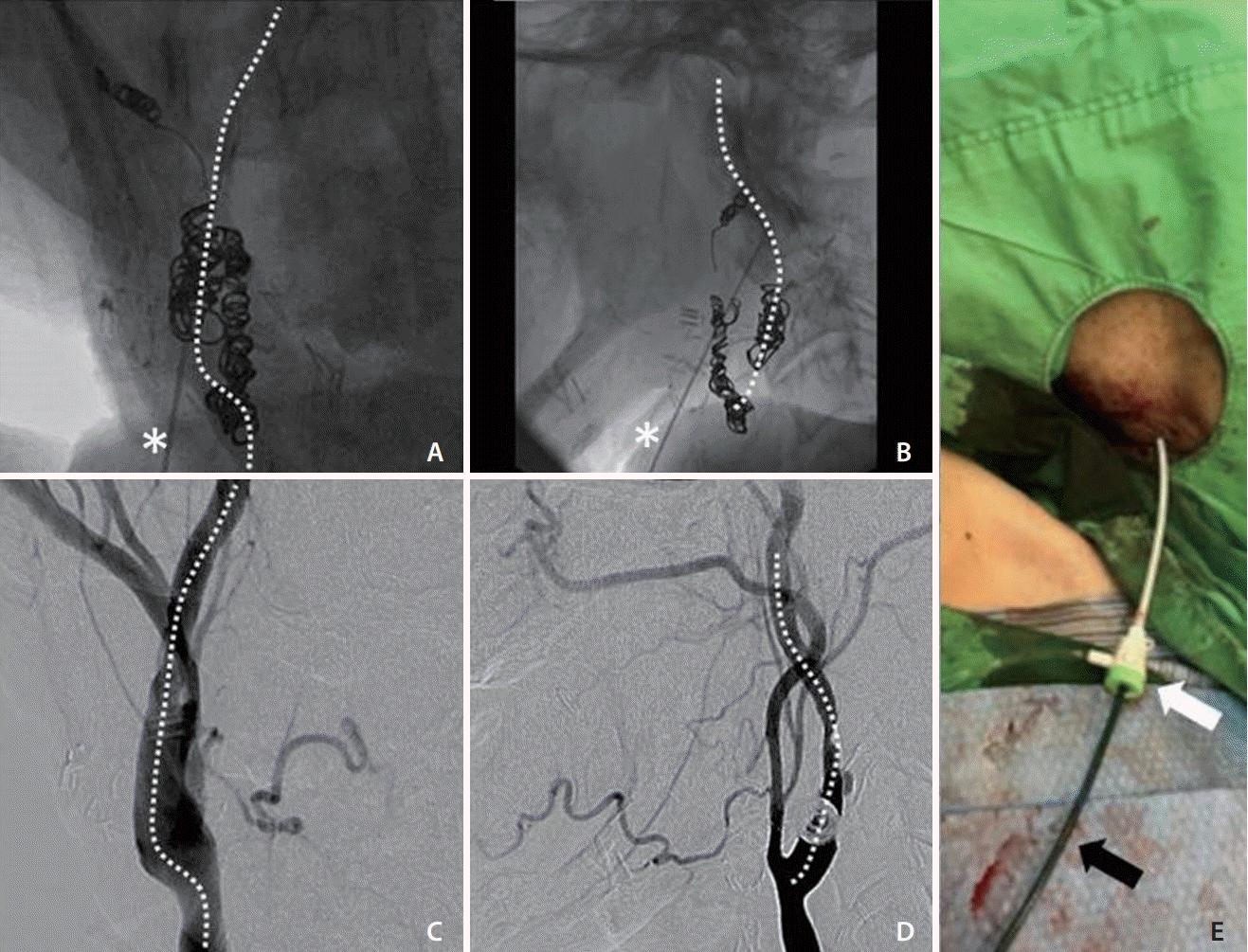
Fig. 4.
Endovascular mechanical thrombectomy. (A, B) Frontal and lateral views of right ICA angiography showing progressive thrombosis in the right ICA and total occlusion of the MCA. The tip of the guiding catheter (white arrows) was placed in the upper cervical portion of the ICA, and the angiograms were obtained via the intermediate catheter at the distal petrous portion (black arrows). (C, D) Frontal and lateral views of right ICA angiography after suction thrombectomy showing TICI grade 3 reperfusion. ICA, internal carotid artery; MCA, middle cerebral artery; TICI, Thrombolysis in Cerebral Infarction.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download