Abstract
Purpose
Although endovascular treatment is currently thought to only be suitable for patients who have pial arterial filling scores >3 as determined by multiphase computed tomography angiography (mpCTA), a cut-off score of 3 was determined by a study, including patients within 12 hours after symptom onset. We aimed to investigate whether a cut-off score of 3 for endovascular treatment within 6 hours of symptom onset is an appropriate predictor of good functional outcome at 3 months.
Materials and Methods
From April 2015 to January 2016, acute ischemic stroke patients treated with mechanical thrombectomy within 6 hours of symptom onset were enrolled into this study. Pial arterial filling scores were semi-quantitatively assessed using mpCTA, and clinical and radiological parameters were compared between patients with favorable and unfavorable outcomes. Multivariate logistic regression analysis was then performed to investigate the independent association between clinical outcome and pial collateral score, with the predictive power of the latter assessed using C-statistics.
Results
Of the 38 patients enrolled, 20 (52.6%) had a favorable outcome and 18 had an unfavorable outcome, with the latter group showing a lower mean pial arterial filling score (3.6±0.8 vs. 2.4±1.2, P=0.002). After adjusting for variables with a P-value of <0.1 in univariate analysis (i.e., age and National Institutes of Health Stroke Scale score at admission), pial arterial filling scores higher than a cut-off of 2 were found to be independently associated with favorable clinical outcomes (P=0.012). C-statistic analysis confirmed that our model had the highest prediction power when pial arterial filling scores were dichotomized at >2 vs. ≤2.
Endovascular treatment in patients with acute ischemic stroke has evolved substantially in recent years. Five randomized clinical trials have led to the establishment of endovascular treatment using stent retriever devices as the standard of care for patients with proximal anterior circulation occlusion [1-3].
Some studies have shown that the degree of leptomeningeal or pial collateral circulation on single-phase computed tomography angiography (spCTA) is associated with clinical outcome, infarct volume, hemorrhage transformation risk, and recanalization rates in acute stroke patients [4-6] and infarct volume and clinical outcome in patients undergoing thrombolysis [7]. Moreover, collateral circulation on dynamic CTA predicts clinical outcome at 3 months [8]. However, although spCTA and dynamic CTA are widely used for pial collateral status evaluation for acute stroke, spCTA lacks temporal resolution and may mislabel pial collateral status, and dynamic CTA needs postprocessing. An alternative is multiphase computed tomography angiography (mpCTA), which as a time-resolved technique can give clinicians more accurate information on the degree and extent of collaterals. Indeed, mpCTA shows better interrater reliability and is associated with better clinical outcomes than spCTA when used to assess acute stroke patients, can be quickly performed, and yields images that can be interpreted without specialist assistance [9,10].
The degree of pial collateral circulation on mpCTA is measured by the 6-point pial arterial filling score, with patients scoring 0–3 not likely to benefit from recanalization with a proximal intracranial occlusion in the anterior circulation admitted within 12 hours after symptom onset [9]. However, we thought a different decision strategy using collateral status is needed for patients with a proximal intracranial occlusion in the anterior circulation admitted within 6 hours after symptom onset. Therefore, we aimed to investigate whether the current pial arterial filling score dichotomization of 0–3 vs. 4–5 is appropriate in triaging patients for endovascular treatment with acute ischemic stroke and large vessel occlusion in the anterior circulation who were admitted within 6 hours after symptom onset.
The Seoul National University Bundang Hospital Institutional Review Board (IRB No. B-1608-357-115) approved this retrospective study, and informed consent was waived.
This study was a retrospective analysis of data prospectively collected from acute ischemic stroke patients who presented at our institution within 6 hours of symptom onset from April 2015 to January 2016. Patients were included if they met the following inclusion criteria: 1) baseline imaging data, including non-contrast computed tomography and mpCTA prior to endovascular treatment, were available, 2) there was occlusion of the intracranial segment of the internal carotid artery (ICA) or middle cerebral artery (MCA) (M1 or M2 segment), and 3) they had a National Institutes of Health Stroke Scale (NIHSS) score of 5 or higher. We excluded patients with poor-quality mpCTA data that prevented the assessment of pial collateral circulation and those who were lost to follow-up.
All patients underwent a standard scanning protocol at presentation using a 256-slice multidetector CT scanner (Brilliance iCT 256; Philips Medical Systems, Cleveland, OH, USA). The mpCTA scanning parameters used were 80 kVp, 175 mAs, and 128×0.625 mm detector collimation. A total of 90 mL of contrast was injected for the scan at a rate of 4–5 mL/sec, followed by a 30-mL normal saline chase at a rate of 3 mL/sec.
The first phase of imaging involved an aortic arch-to-vertex CTA scan that was initiated by the bolus tracking method with a trigger level of 150 Hounsfield units at the aortic arch, minimum scan delay, and an average dose length product of 1,100 to 1,200 mGy∙cm. This was acquired in less than 3 seconds. The second phase started after a delay of 4 seconds and was acquired in 3 seconds from the vertex to skull base. The third phase was initiated after another 4-second delay with scanning in the opposite direction from the skull base to vertex. The scan was performed in this direction for the third phase because our scanner is not able to be repositioned within the time limit of 7 seconds between phases. Images from the first phase (i.e., the aortic arch-tovertex scan) were then reconstructed as 1.5-mm-thick axial sections. In addition, 24-mm-thick slab multi-planar reformations of axial first to third phase images from the skull base to vertex were performed to assess the degree of pial collateral filling.
Radiologic data were independently assessed by a neurologist (H.G.W.) and 2 neuroradiologists (C.J. and L.S.) who were blinded to patient clinical symptoms. Pial arterial filling score was measured in accordance with a previously published report [9]. Although there was excellent interrater reliability between the two radiologists (n=30, κ=0.81, P<0.001) in a previous report, interrater reliability in our study was moderate on first interpretation (n=38, κ=0.46, P<0.001). Hence, we needed to clarify the three determinants of pial arterial filling score, i.e., the degree of phase delay, prominence, and extent of pial collaterals. The degree of phase delay was defined as the difference in the phase of pial collateral filling between thick axial maximum intensity projections of the head in the index territory and that in the contralateral territory [12]. Reductions in the prominence and extent of pial collaterals were defined as when the number of pial vessels in the index territory was smaller than that in the contralateral territory, with adjustment for different phases of collateral filling between the index and contralateral territories. Ischemic regions with no vessels were defined as regions where one third or more of the lesions in the index territory had no collateral filling in any phase (Table 1).
A neurointerventionalist (C.J.) performed intraarterial treatment (IAT) of all the cases on a biplane angiography machine (Integris Allura, Philips, the Netherlands). We used a 9 French balloon guiding catheter. In case of a tortuous cervical ICA, a second guiding catheter was used for the coaxial system. The primary device for IAT was a stent retriever (Solitaire FR; Covidien, Irvine, California; Trevo-XP; Stryker, Kalamazoo, MI, USA). Mechanical aspiration device, thrombolytics, or permanent stenting were also used at the discretion of operator. The reperfusion grading was measured by using modified treatment in cerebral infarction before and after IAT [13].
Patient demographics, as well as clinical and radiological findings, were compared between patients with favorable (modified Rankin Scale [mRS] at 3 months ≤2) and unfavorable (mRS at 3 months >2) outcomes using the Pearson chi-square test, independent t test, and Mann-Whitney U test as appropriate. Univariate and multivariate logistic regression analyses were then performed to identify factors associated with a favorable functional outcome. Variables with a P-value of ≤0.1 in univariate analysis were included in multivariate analyses. We then determined the area under the receiver-operating characteristic curve (AUC) to assess model fit and compared AUC values between pial arterial filling scores dichotomized at various cut-off scores (i.e., >4 vs. ≤4, >3 vs. ≤3, and >2 vs. ≤2). Areas under the receiver operating characteristic curves for these 2 scoring systems were compared using DeLong’s test. All statistical analyses were performed using SPSS Statistics 22 (IBM, Armonk, NY, USA) and Stata 13.0 software (Stata Corporation, College Station, TX, USA). P values <0.05 were considered statistically significant. Results are expressed as mean±standard deviation where appropriate.
Of the 838 patients in our database with acute ischemic stroke, 257 patients underwent mpCTA within 6 hours of symptom onset. Among these, 208 patients who were not candidates for endovascular revascularization were excluded due to mild symptoms (NIHSS <4, 152 patients), no occlusion identified on mpCTA (46 patients), and hemorrhagic diathesis (10 patients). In total, 11 patients underwent endovascular treatment for occlusion in the basilar artery or vertebra artery, and 38 underwent endovascular treatment using a stent retriever for ICA/MCA occlusion identified on mpCTA. The mean age of enrolled patients was 72.4±10.2 years, and 55.3% were male. Favorable outcomes were observed in 20 patients (52.6%), whereas unfavorable outcomes were observed in 18 (47.4%). The most common stroke subtype was cardioembolism (63.2%). Thirty-one patients (81.6%) were treated with intravenous tissue plasminogen activator before endovascular treatment, and 34 (89.5%) had successful reperfusion. The mean pial arterial filling score was 3.0±1.2.
Interrater reliability of the pial arterial filling score between a neurologist and two neuroradiologists, as assessed using unweighted k statistics with 95% confidence intervals (CIs), indicated substantial agreement (n=38, κ=0.64, P<0.001).
Patient demographics and clinical and radiological parameters are shown in Table 2. Mean age was lower in the favorable (67.1±10.4 years) than in the unfavorable (78.3±6.0 years) outcome groups (P<0.001), while pial arterial filling score was higher in the favorable (3.6±0.8) than in the unfavorable (2.4±1.2) outcome groups (P=0.002).
In our univariate analysis, younger age (odds ratio [OR], 0.825; 95% CI, 0.720–0.945; P=0.006) and lower NIHSS score at admission (OR, 0.839; 95% CI, 0.720–0.977; P=0.024) were significantly associated with favorable outcomes at 3 months. Higher pial arterial filling score (OR, 2.917; 95% CI, 0.321–6.443; P=0.008) was also significantly associated with favorable outcomes. Because of the ambiguity of the score threshold for predicting clinical outcomes in endovascular treatment, we dichotomized the pial arterial filling score at >4 vs. ≤4, >3 vs. ≤3, and >2 vs. ≤2 and used each dichotomization as a predictor variable in our analyses. Similar trends to those above were shown when the pial arterial filling score was dichotomized at >2 vs. ≤2 (OR, 14.143; P=0.003). In contrast, pial arterial filling scores dichotomized at >3 vs. ≤3 and >4 vs. ≤4 were not significantly associated with clinical outcome. After adjusting for age and NIHSS score at admission, higher pial arterial filling scores and pial arterial filling scores dichotomized at >2 vs. ≤2 were found to be independently associated with favorable clinical outcomes (Table 3). The pial arterial filling scores dichotomized at >2 vs. ≤2 (AUC, 0.756; 95% CI, 0.621–0.89; P=0.249) also effectively predicted favorable clinical outcomes at 3 months, with higher AUC than dichotomization at pial arterial filling scores dichotomized at >3 vs. ≤3 and >4 vs. ≤4 (AUC, 0.636; 95% CI, 0.482–0.791; P=0.364, and AUC, 0.550; 95% CI, 0.483–0.617; P<0.001, respectively) (Fig. 2). The difference was statistically significant in favor of the pial arterial filling scores dichotomized at >2 vs. ≤2 compared with the pial arterial filling scores dichotomized at >4 vs. ≤4 (DeLong’s test, P-value=0.006). However, the difference was not statistically significant between the pial arterial filling scores dichotomized at >3 vs. ≤3 compared with the pial arterial filling scores dichotomized at >4 vs. ≤4 (DeLong’s test, P-value=0.274) and between the pial arterial filling scores dichotomized at >2 vs. ≤2 compared with the pial arterial filling scores dichotomized at >3 vs. ≤3 (DeLong’s test, P-value=0.073).
We found that pial arterial filling scores were higher in patients with favorable outcomes than in those with unfavorable outcomes. In particular, a pial arterial filling score cut-off of 2 on mpCTA was significantly associated with favorable clinical outcomes at 3 months after endovascular treatment for acute ischemic stroke with large vessel occlusion of the anterior circulation within 6 hours since last seen normal.
The appropriate pial arterial filling score cut-off for determining tissue salvageability and selecting suitable candidates for endovascular treatment has not been externally validated, although recommendations have been previously made. Specifically, the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) trial demonstrated that moderate to good collateral circulation leads to decreased mortality and significantly improved clinical outcome after endovascular treatment [14]. For decision making with mpCTA, the ESCAPE trial considered a pial arterial filling score of >3 to indicate the presence of salvageable brain; thus, scores of 0–3 were considered exclusion criteria for endovascular treatment [14]. Furthermore, in the Interventional Management of Stroke trial, the rate of favorable outcomes in patients with poor collaterals treated with endovascular therapy ranged from only 5.3% to 20.6% when they were assessed using three different ordinal scoring systems [15]. Although criteria for candidate of endovascular treatment in our institution did not include degree of pial collateral circulation, our external validation of the appropriate cut-off score for determining collateral status using mpCTA within 6 hours since last seen normal suggests that a cut-off score of 2 is more suitable than that of 3 or 4. The reason might be because our study, which included patients with a proximal intracranial occlusion in the anterior circulation that presented within 6 hours after symptom onset, differs from the ESCAPE trial, and because patients with a collateral score of 3 have sufficient collateral perfusion and reversible brain tissues and may thus be good candidates for endovascular revascularization within 6 hours since last seen normal. Practically, as a noninvasive collateral assessment tool for the effectiveness of endovascular treatment in acute ischemic stroke with large vessel occlusion, application of this newly identified pial collateral score cut-off may result in favorable clinical outcomes.
Despite these insights, there are limitations to our study that need to be taken into consideration when interpreting our data. The present study was a single-center study with a limited number of patients. Furthermore, a previous report showed better interrater reliability to assess pial arterial filling scores than the present study. However, for a reduced time interval from within 12 hours since last seen normal to within 6 hours since last seen normal, a smaller number of patients can be expected. Therefore, further studies with larger patient cohorts need to be conducted. Additionally, the timing of the CTA contrast injection relative to imaging is likely to be critical [16]. If CTA images are acquired too early, different phases may fail to be measured, leading to overestimation of the pial arterial filling score. As such, poor cardiac function can also interfere with pial arterial filling, as shown on mpCTA.
Pial arterial filling scores dichotomized at ≤2 vs. >2 as determined by mpCTA for a 6-hour time window appear to be suitable for predicting clinical outcome at 3 months after mechanical thrombectomy for acute ischemic stroke with large vessel occlusion of the anterior circulation. Also, critical re-appraisal for the cut-off score of mpCTA should be re-assessed by well-designed further trials.
REFERENCES
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.

2. Hong KS, Ko SB, Yu KH, Jung C, Park SQ, Kim BM, et al. Update of the korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2016; 18:102–113.

3. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:3020–3035.

4. Liebeskind DS. Collateral lessons from recent acute ischemic stroke trials. Neurol Res. 2014; 36:397–402.

5. Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol. 2009; 30:165–170.

6. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011; 42:693–699.

7. Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005; 26:1789–1797.
8. van den Wijngaard IR, Boiten J, Holswilder G, Algra A, Dippel DW, Velthuis BK, et al. Impact of collateral status evaluated by dynamic computed tomographic angiography on clinical outcome in patients with ischemic stroke. Stroke. 2015; 46:3398–3404.

9. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase ct angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015; 275:510–520.

10. Yu AY, Zerna C, Assis Z, Holodinsky JK, Randhawa PA, Najm M, et al. Multiphase ct angiography increases detection of anterior circulation intracranial occlusion. Neurology. 2016; 87:609–616.

11. Yang CY, Chen YF, Lee CW, Huang A, Shen Y, Wei C, et al. Multiphase ct angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol. 2008; 29:1288–1295.

12. Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke. 2016; 47:273–281.
13. Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013; 44:2509–2512.

14. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.
15. Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, et al. Differential effect of baseline computed tomographic angiography collaterals on clinical outcome in patients enrolled in the interventional management of stroke iii trial. Stroke. 2015; 46:1239–1244.

16. Mortimer AM, Simpson E, Bradley MD, Renowden SA. Computed tomography angiography in hyperacute ischemic stroke: prognostic implications and role in decision-making. Stroke. 2013; 44:1480–1488.
Fig. 1.
Pial arterial filling score within the symptomatic ischemic territory using multiphase CT angiography images. Top row: images of a patient with a left MCA M1 segment occlusion (arrow) and no delay of phase in filling in of peripheral vessels, but with the same prominence and extent. Second row: images of a patient with a right MCA M1 segment occlusion (arrow) and a delay of 1 phase in filling in of peripheral vessels, but with the same prominence and extent. Third row: images of a patient with a left MCA M1 segment occlusion (arrow) and a delay of 2 phases in filling in of peripheral vessels, but with the same prominence and extent. Fourth row: images of a patient with a right MCA M1 segment occlusion (arrow) and a delay of 1 phase in filling in of peripheral vessels, but with decreased prominence and extent. Fifth row: images of a patient with a right MCA M1 segment occlusion (arrow) and just a few vessels visible in any phase within the occluded vascular territory. Bottom row: images of a patient with a left MCA M1 segment occlusion (arrow) and no vessels visible in any phase within the ischemic vascular territory. CT, computed tomograpy; MCA, middle cerebral artery.
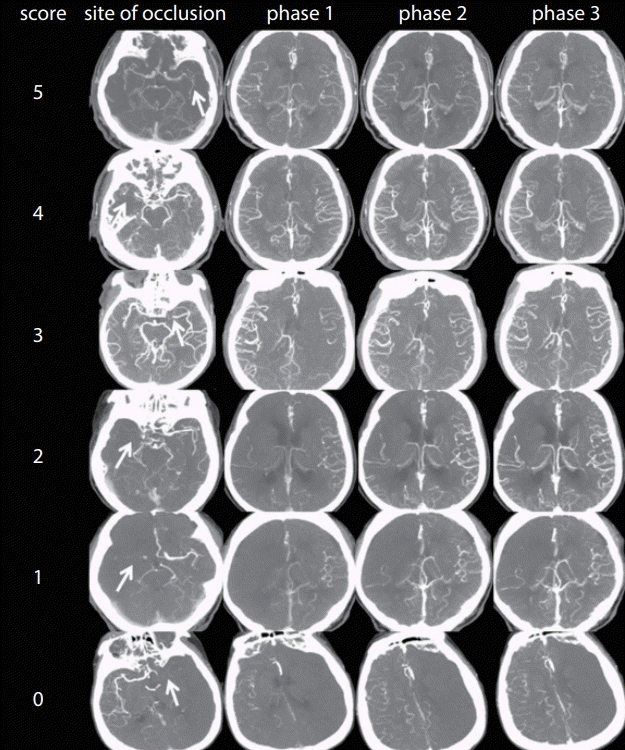
Fig. 2.
Results of receiver operating characteristic analysis for investigating functional outcome using each dichotomized pial arterial filling score (>4 vs. ≤4, >3 vs. ≤3, and >2 vs. ≤2). AUC, area under the receiver-operating characteristic curve; CI, confidence interval.
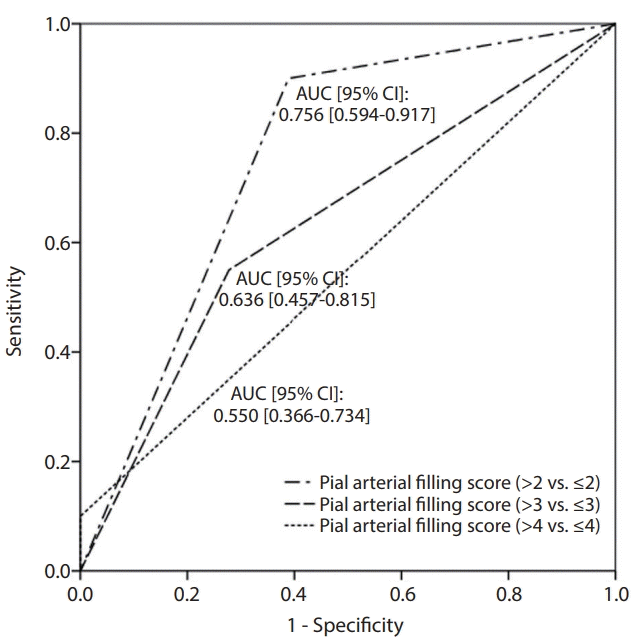
Table 1.
Pial arterial filling score used in the current study within the symptomatic ischemic territory compared with the asymptomatic contralateral hemisphere using multiphase CT angiography
Table 2.
Comparison of demographics and clinical and radiological parameters according to clinical outcome at 3 months after stroke
Table 3.
Association factors for favorable outcome at 3 months after stroke
| OR (95% CI) | P-value | Adjusted* OR (95% CI) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 0.825 (0.720–0.945) | 0.006 | ||
| Male sex | 2.321(0.628–8.579) | 0.207 | ||
| Risk factor | ||||
| Diabetes mellitus | 0.667 (0.163–2.727) | 0.573 | ||
| Hypertension | 2.918 (0.780–10.924) | 0.112 | ||
| Atrial fibrillation | 0.470 (0.121–1.825) | 0.275 | ||
| Smoking | 0.313 (0.074–1.315 | 0.113 | ||
| Hyperlipidemia | 1.500 (0.346–6.498) | 0.588 | ||
| Previous stroke | 2.000 (0.320–12.510) | 0.459 | ||
| NIHSS score at admission | 0.839 (0.720–0.977) | 0.024 | ||
| Intravenous thrombolysis | 0.375 (0.063–2.234) | 0.281 | ||
| Onset to reperfusion time (minutes) | 1.002 (0.996–1.009) | 0.462 | ||
| Procedure time (minutes) | 0.987 (0.966–1.008) | 0.224 | ||
| mTICI (2B or 3) | 3.800 (0.358–40.336) | 0.268 | ||
| Pial arterial filling score | 2.917 (0.321–6.443) | 0.008 | 3.724 (1.141–12.156) | 0.029 |
| Dichotomized score (>3 vs. ≤3) | 3.718 (0.819–12.337) | 0.095 | 2.374 (0.380–14.814) | 0.355 |
| Dichotomized score (>2 vs. ≤2) | 14.143 (2.479–80.682) | 0.003 | 84.893 (2.680–2689.571) | 0.012 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download