1. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011; 42(1 Suppl):s20–s23.
2. Kim JS, Caplan LR, Wong KSL. Intracranial atherosclerosis. Oxford: John Wiley & Sons;2009.
3. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The northern manhattan stroke study. Stroke. 1995; 26:14–20.
4. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008; 39:2396–2399.
5. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006; 1:158–159.

6. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005; 352:1305–1316.

7. Kim DE, Lee KB, Jang IM, Roh H, Ahn MY, Lee J. Associations of cigarette smoking with intracranial atherosclerosis in the patients with acute ischemic stroke. Clin Neurol Neurosurg. 2012; 114:1243–1247.

8. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986; 17:648–655.

9. Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci. 2009; 284:40–45.

10. Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012; 43:3313–3318.
11. Willeit J, Kiechl S. Prevalence and risk factors of asymptomatic extracranial carotid artery atherosclerosis. A population-based study. Arterioscler Thromb. 1993; 13:661–668.

12. Ding X, Li C, Yu K, Gao A, Xiao L, Peng F, et al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: a systematic review and meta-analysis. Int J Neurosci. 2014; 124:834–840.

13. Kwak JH, Zhao L, Kim JK, Park S, Lee DG, Shim JH, et al. The outcome and efficacy of recanalization in patients with acute internal carotid artery occlusion. AJNR Am J Neuroradiol. 2014; 35:747–753.

14. Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated mca disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. 2015; 46:697–703.
15. Song Y, Kim JG, Cho HJ, Kim JK, Suh DC. Evaluation of cerebral blood flow change after cigarette smoking using quantitative MRA. PLoS One. 2017; 12:e0184551.

16. D’Armiento FP, Bianchi A, de Nigris F, Capuzzi DM, D’Armiento MR, Crimi G, et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke. 2001; 32:2472–2479.
17. Lü PH, Park JW, Park S, Kim JL, Lee DH, Kwon SU, et al. Intracranial stenting of subacute symptomatic atherosclerotic occlusion versus stenosis. Stroke. 2011; 42:3470–3476.

18. Suh DC, Kim JK, Choi JW, Choi BS, Pyun HW, Choi YJ, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol. 2008; 29:781–785.

19. Suh DC, Kim JK, Choi CG, Kim SJ, Pyun HW, Ahn C, et al. Prognostic factors for neurologic outcome after endovascular revascularization of acute symptomatic occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2007; 28:1167–1171.

20. Choi JW, Kim JK, Choi BS, Kim JH, Hwang HJ, Kim JS, et al. Adjuvant revascularization of intracranial artery occlusion with angioplasty and/or stenting. Neuroradiology. 2009; 51:33–43.

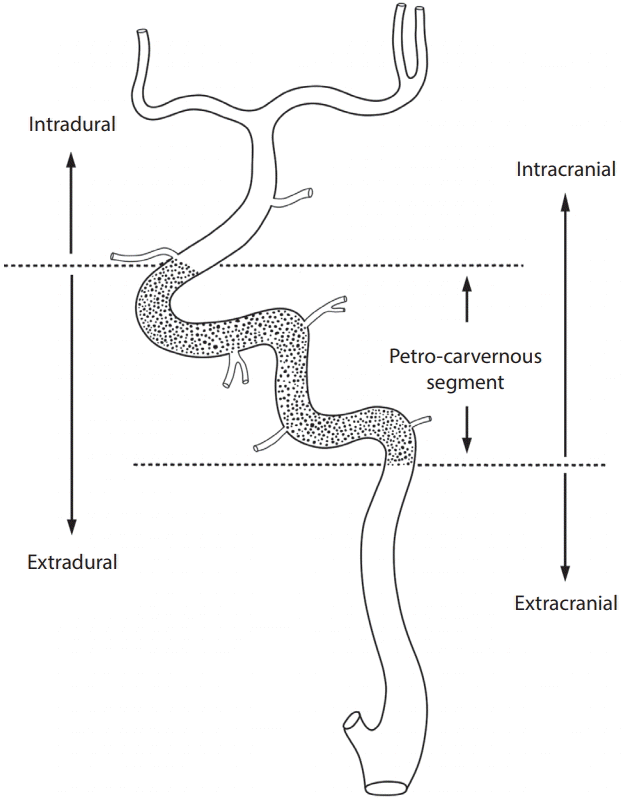
21. Lasjaunias P, Berenstein A, Brugge KT. Skull base and maxillo-facial region. Surgical neuroangiography. 2nd ed. Berlin: Springer-verlag;2001. p. 261–290.
22. Willinek WA, von Falkenhausen M, Born M, Gieseke J, Höller T, Klockgether T, et al. Noninvasive detection of steno-occlusive disease of the supra-aortic arteries with three-dimensional contrast-enhanced magnetic resonance angiography: a prospective, intra-individual comparative analysis with digital subtraction angiography. Stroke. 2005; 36:38–43.
23. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000; 21:643–646.
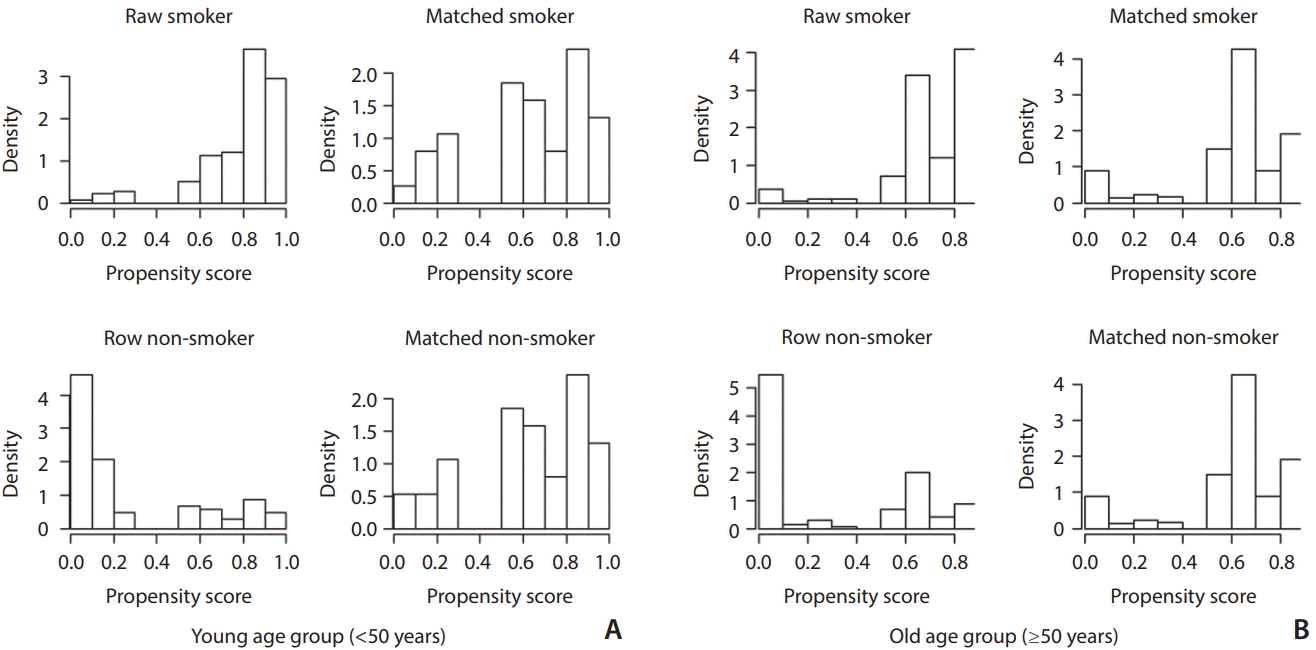
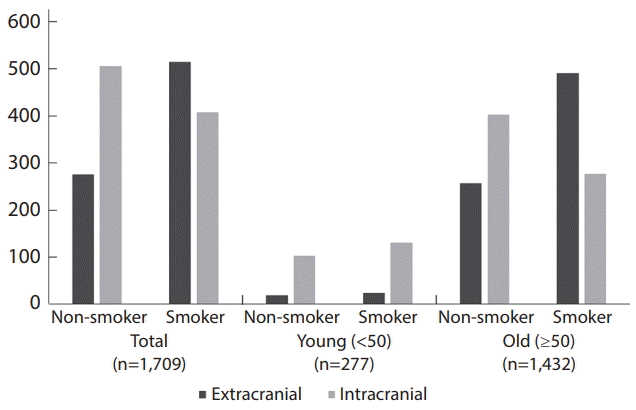
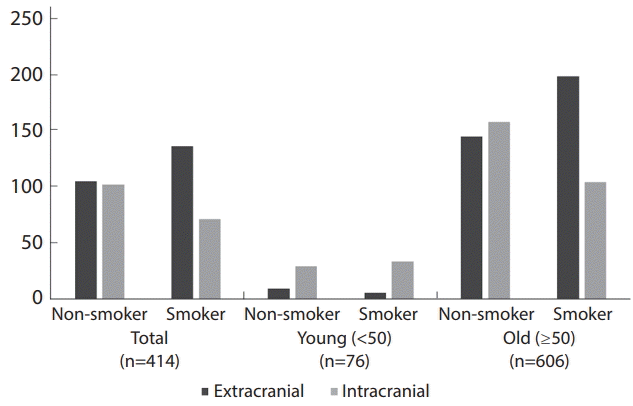
24. Liu H, Lee DG, Jung SC, Koo HJ, Kim EH, Hwang SM, et al. A study design to evaluate association between smoking and intracranial atherosclerotic stenosis. Neurointervention. 2014; 9:89–93.

25. Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005; 94:1–12.

26. Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014; (260):1–161.
27. Ji R, Pan Y, Yan H, Zhang R, Liu G, Wang P, et al. Current smoking is associated with extracranial carotid atherosclerotic stenosis but not with intracranial large artery disease. BMC Neurol. 2017; 17:120.

28. Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, et al. Consensus conference on intracranial atherosclerotic disease: rationale, methodology, and results. J Neuroimaging. 2009; 19 Suppl 1:1S. 10S.

29. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke. 2014; 16:8–17.

30. Kim YD, Choi HY, Jung YH, Nam CM, Yang JH, Cho HJ, et al. Classic risk factors for atherosclerosis are not major determinants for location of extracranial or intracranial cerebral atherosclerosis. Neuroepidemiology. 2009; 32:201–207.

31. Siddiq F, Chaudhry SA, Vazquez G, Suri MF, Qureshi AI. Intracranial stenosis in young patients: unique characteristics and risk factors. Neuroepidemiology. 2012; 38:148–153.

32. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014; 45:663–669.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download