1. Codman EA. The shoulder: rupture of the suprasupinatus tendon. Boston: Todd Company;1934. p. 216–224.
2. Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. J Bone Joint Surg Am. 1945; 27:211–222.
3. Ha'eri GB, Maitland A. Arthroscopic findings in the frozen shoulder. J Rheumatol. 1981; 8:149–152. PMID:
7218244.
4. Kilian O, Kriegsmann J, Berghauser K, Stahl JP, Horas U, Heerdegen R. The frozen shoulder: arthroscopy, histological findings and transmission electron microscopy imaging. Chirurg. 2001; 72:1303–1308. PMID:
11766655.
5. Uitvlugt G, Detrisac DA, Johnson LL, Austin MD, Johnson C. Arthroscopic observations before and after manipulation of frozen shoulder. Arthroscopy. 1993; 9:181–185. PMID:
8461078.

6. Wiley AM. Arthroscopic appearance of frozen shoulder. Arthroscopy. 1991; 7:138–143. PMID:
2069623.

7. Hannafin JA, Chiaia TA. Adhesive capsulitis: a treatment approach. Clin Orthop Relat Res. 2000; (372):95–109. PMID:
10738419.
8. Mitra R, Harris A, Umphrey C, Smuck M, Fredericson M. Adhesive capsulitis: a new management protocol to improve passive range of motion. PM R. 2009; 1:1064–1068. PMID:
20006315.

9. Simpson JK, Budge R. Treatment of frozen shoulder using distension arthrography (hydrodilatation): a case series. Australas Chiropr Osteopathy. 2004; 12:25–35. PMID:
17987207.
10. Andren L, Lundberg BJ. Treatment of rigid shoulders by joint distension during arthrography. Acta Orthop Scand. 1965; 36:45–53. PMID:
14308098.
11. Haines JF, Hargadon EJ. Manipulation as the primary treatment of the frozen shoulder. J R Coll Surg Edinb. 1982; 27:271–275. PMID:
7143295.
12. Ekelund AL, Rydell N. Combination treatment for adhesive capsulitis of the shoulder. Clin Orthop Relat Res. 1992; (282):105–109. PMID:
1516300.

13. Tveita EK, Tariq R, Sesseng S, Juel NG, Bautz-Holter E. Hydrodilatation, corticosteroids and adhesive capsulitis: a randomized controlled trial. BMC Musculoskelet Disord. 2008; 9:53. PMID:
18423042.

14. Oh JH, Oh CH, Choi JA, Kim SH, Kim JH, Yoon JP. Comparison of glenohumeral and subacromial steroid injection in primary frozen shoulder: a prospective, randomized short-term comparison study. J Shoulder Elbow Surg. 2011; 20:1034–1040. PMID:
21816628.

15. Cole BF, Peters KS, Hackett L, Murrell GA. Ultrasound-guided versus blind subacromial corticosteroid injections for subacromial impingement syndrome: a randomized, double-blind clinical trial. Am J Sports Med. 2016; 44:702–707. PMID:
26717970.
16. Kim YS, Lee HJ, Lee DH, Choi KY. Comparison of high- and low-dose intra-articular triamcinolone acetonide injection for treatment of primary shoulder stiffness: a prospective randomized trial. J Shoulder Elbow Surg. 2017; 26:209–215. PMID:
27914846.

17. Kim JS, Kwon JY, Lee WI, Kim JM. The additional effect of tear after passive exercise during distension arthrography in patients with frozen shoulder. J Korean Acad Rehabil Med. 2008; 32:324–326.
18. Roubal PJ, Dobritt D, Placzek JD. Glenohumeral gliding manipulation following interscalene brachial plexus block in patients with adhesive capsulitis. J Orthop Sports Phys Ther. 1996; 24:66–77. PMID:
8832469.

19. Cyriax JH. Textbook of orthopaedic medicine (Volume 1. Diagnosis of soft tissue lesions). 7th ed. London: Harcourt Publisher;1978. p. 224–225.
20. Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract. 2003; 3:310–316. PMID:
17166126.

21. Gam AN, Schydlowsky P, Rossel I, Remvig L, Jensen EM. Treatment of “frozen shoulder” with distension and glucorticoid compared with glucorticoid alone: a randomised controlled trial. Scand J Rheumatol. 1998; 27:425–430. PMID:
9855212.
22. Piotte F, Gravel D, Moffet H, Fliszar E, Roy A, Nadeau S, et al. Effects of repeated distension arthrographies combined with a home exercise program among adults with idiopathic adhesive capsulitis of the shoulder. Am J Phys Med Rehabil. 2004; 83:537–546. PMID:
15213478.

23. Lundberg BJ. The frozen shoulder: clinical and radiographical observations; the effect of manipulation under general anesthesia; structure and glycosaminoglycan content of the joint capsule; and local bone metabolism. Acta Orthop Scand Suppl. 1969; 119:1–59. PMID:
4952729.
24. Mao CY, Jaw WC, Cheng HC. Frozen shoulder: correlation between the response to physical therapy and follow-up shoulder arthrography. Arch Phys Med Rehabil. 1997; 78:857–859. PMID:
9344306.

25. Placzek JD, Roubal PJ, Freeman DC, Kulig K, Nasser S, Pagett BT. Long-term effectiveness of translational manipulation for adhesive capsulitis. Clin Orthop Relat Res. 1998; (356):181–191. PMID:
9917683.
26. Amoretti N, Grimaud A, Brocq O, Roux C, Dausse F, Fournol M, et al. Shoulder distension arthrography in adhesive capsulitis. Clin Imaging. 2006; 30:254–256. PMID:
16814141.

27. Loew M, Heichel TO, Lehner B. Intraarticular lesions in primary frozen shoulder after manipulation under general anesthesia. J Shoulder Elbow Surg. 2005; 14:16–21. PMID:
15723009.

28. Choi JK, Son SB, Park BJ, Yang SN, Yoon JS. Shoulder manipulation after distention arthrography: does audible cracking affect improvement in adhesive capsulitis? A preliminary study. Ann Rehabil Med. 2015; 39:745–751. PMID:
26605172.

29. Flannery O, Mullett H, Colville J. Adhesive shoulder capsulitis: does the timing of manipulation influence outcome? Acta Orthop Belg. 2007; 73:21–25. PMID:
17441653.
30. van Royen BJ, Pavlov PW. Treatment of frozen shoulder by distension and manipulation under local anaesthesia. Int Orthop. 1996; 20:207–210. PMID:
8872541.

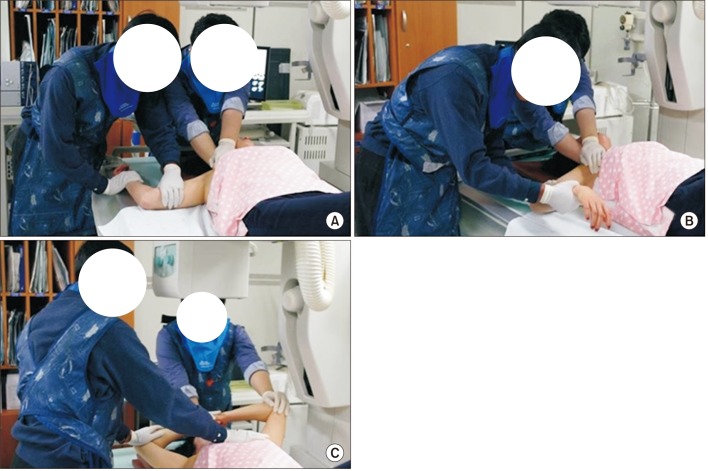
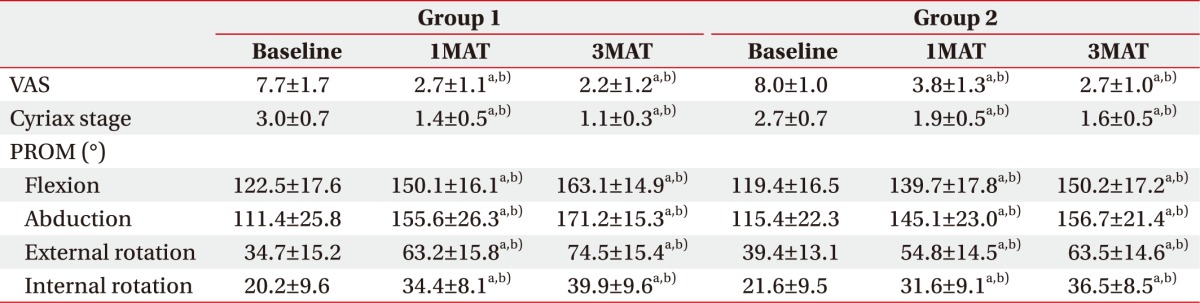
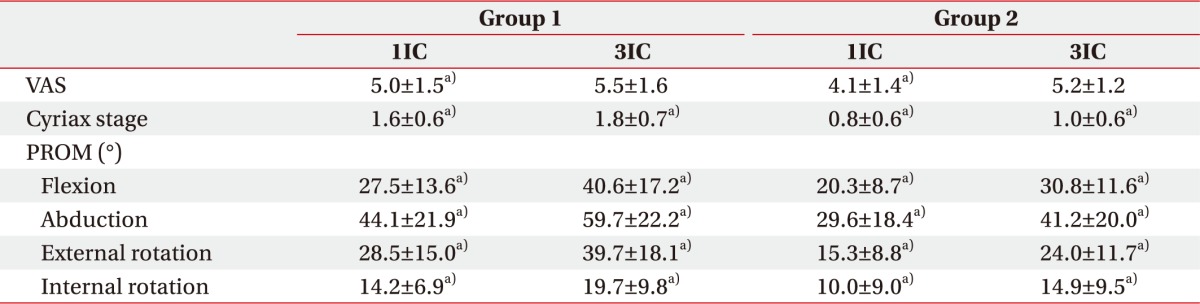




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download