This article has been
cited by other articles in ScienceCentral.
Abstract
Hennekam syndrome is a rare autosomal recessive disorder resulting from malformation of the lymphatic system. The characteristic signs of Hennekam syndrome are lymphangiectasia, lymph edema, facial anomalies, and mental retardation. This is a case in which a patient presented with left-arm lymphedema, facial-feature anomalies, and multiple organ lymphangiectasia consistent with symptoms of Hennekam syndrome. There is no curative therapy at this time, but rehabilitative treatments including complete decongestive therapy for edema control appeared to be beneficial.
Go to :

Keywords: Lymphedema, Hennekam lymphangiectasia lymphedema syndrome
INTRODUCTION
Hennekam syndrome is an autosomal recessive disorder resulting from malformation of the lymphatic system. This syndrome was first described by Dutch physician Hennekam in 1989 [
1]. It is a very rare syndrome and fewer than 50 cases have been reported in medical literature [
2].
The characteristic signs of Hennekam syndrome are lymphangiectasia, lymph edema, facial anomalies, and mental retardation [
3]. Malformations of lymphatic channels block the lymph circulation, and accumulation of fluids affects multiple body parts including the face and limbs, as well as internal organs. Facial features are characterized by a flattened appearance of the face, a broad depressed nasal bridge, hypertelorism, epicanthal folds, a small mouth, and other anomalies [
3].
This syndrome is often diagnosed on the basis of clinical symptoms, but more recent studies have been made through genetic analysis. About 25% of patients who have Hennekam syndrome were found to have autosomal recessive mutations in
CCBE1 [
4] and a further 20% of patients have mutations in
FAT4 [
5].
We present the case of a 28-month-old Russian female affected with Hennekam syndrome, who was previously undiagnosed.
Go to :

CASE REPORT
A 28-month-old Russian female presented with congenital left-arm edema. She was born after 38 weeks' gestation by vaginal delivery, and her birth weight was 2.95 kg. She was the second of fraternal twins, but her twin sister displayed no abnormalities. The parents had no history of radiation exposure, the mother had no major illness during pregnancy or abnormal obstetric history. No other family members had similar phenotypic features. She was admitted to a neonatal intensive care unit (NICU) in Russia for treatment of respiratory difficulty and for intense evaluation for 1 month, but no diagnosis was made, and she was eventually referred to our hospital.
At the time of admission, the patient was conscious and her vital signs were within normal limits. Her height was 94 cm (90th centile according to the Center for Disease Control and Prevention [CDC] growth chart) and weight was 14 kg (between 75th–90th centile per the CDC growth chart). She had significant left-arm edema (without pain), limitation of motion, and difficulty with arm movement (
Fig. 1). The circumferences of right and left arms were as follow: upper arm (midpoint between the tip of the olecranon process and the tip of the acromion), 14.5 cm and 22 cm; forearm (at two fingers distance the elbow crease), 14 cm and 22.5 cm, respectively. The amount of water and moisture ratio (i.e., extracellular fluid/total water amount) in the right upper limb was 420 mL and 0.389%, respectively, and those in the left upper limb were 1,050 mL and 0.412%, respectively in bioimpedance analysis (InBody S10; Biospace, Seoul, Korea). A dysmorphic face with flat midface, hypertelorism, depressed nasal bridge, a bulbous nasal tip, and epicanthal folds was also evident (
Fig. 2). All other parts of the body were normal in appearance.
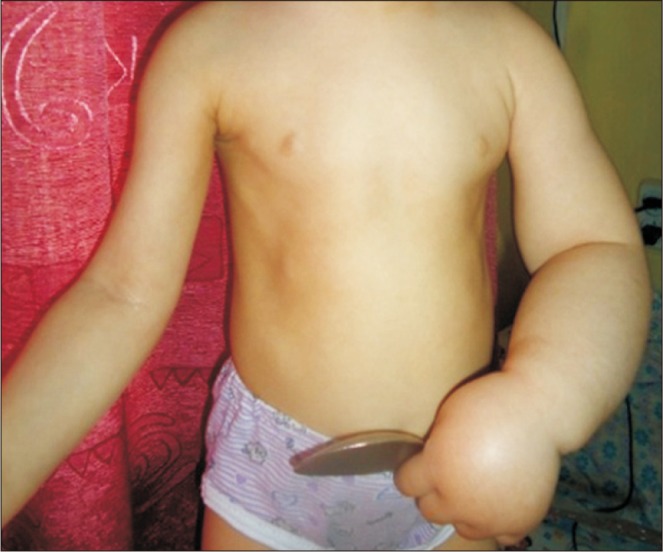 | Fig. 1Lymph edema of left upper extremity.
|
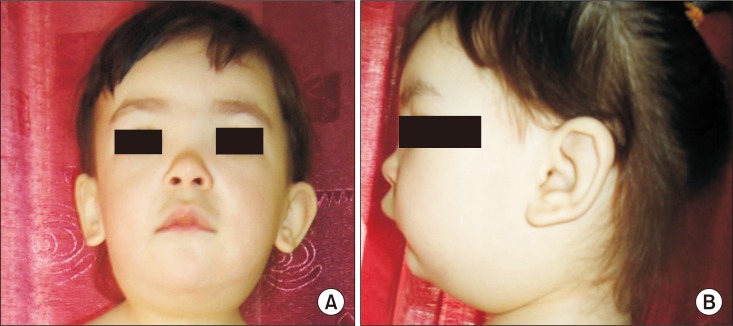 | Fig. 2Dysmorphic face with flat midface, hypertelorism, and broad nasal bridge of front view (A) and side view (B).
|
Various rehabilitative assessment tools were applied, with the help of a Russian interpreter, to evaluate the patient's development. The results showed some developmental delay in gross motor skills, fine motor skills, personal-social functions, and functional activities. Routine blood tests and urinalysis were within normal limits. Total protein (6.1 g/dL) and albumin (4.0 g/dL) levels were also within normal limits.
Based on the characteristic dysmorphic face with congenital lymphatic edema, the possibility of Hennekam syndrome was considered. To identify lymphatic malformations, which were additional typical features of Hennekam syndrome, we used various diagnostic evaluations including X-ray, computed tomography (CT), angiography, lymphatic scan, brain magnetic resonance imaging (MRI), chest CT, and abdomen ultrasonography (US), which indicated lymphatic malformation of multiple organs (
Fig. 3). We diagnosed Hennekam syndrome, based on the clinical features of these various lymphatic abnormalities.
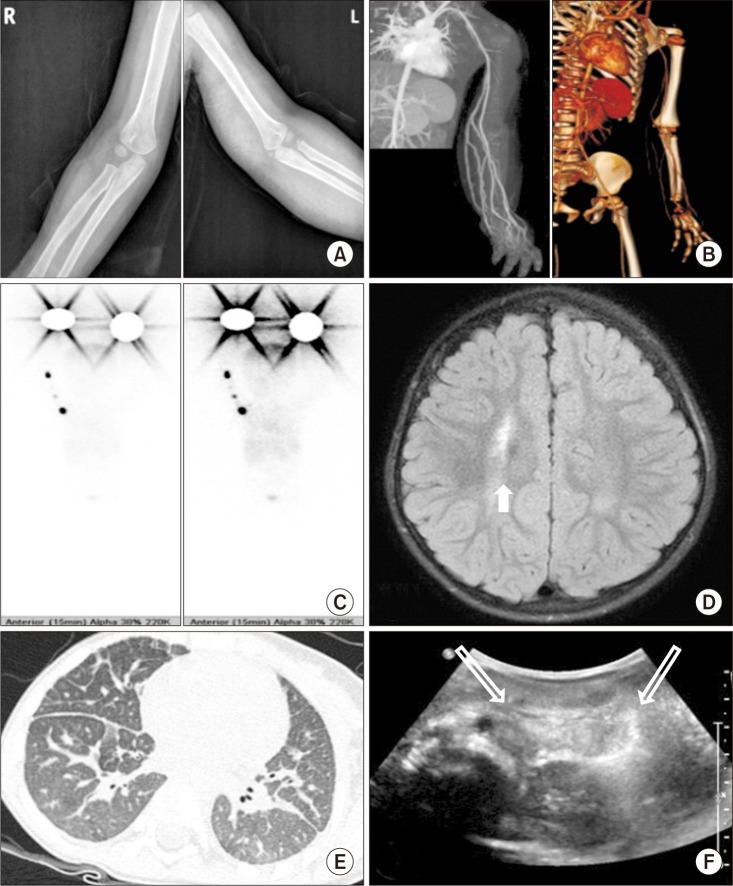 | Fig. 3On diagnostic evaluation of multiple organs, (A) the simple X-ray of both upper extremities showed no gross bony abnormality. (B) The computed tomography (CT) angiography showed a marked lymphedema in the left upper extremity. (C) The lymphatic scan showed no visible lymphatic flow of left upper extremity, which implies primary lymphedema. (D) The brain MRI showed a focal increased signal intensity (arrow). (E) The chest CT showed lymphangiectasia of both lungs. (F) The abdomen ultrasonography showed lymphangiectasia in small bowel mesentery (open arrow).
|
The patient received 7 daily sessions of comprehensive rehabilitative treatment, including activities of daily living training, low-level laser therapy (10-minute application with a wavelength range of 600–1,000 nm), and complete decongestive therapy for lymphedema of left arm. The complete decongestive therapy included manual lymph drainage (10-minute sessions performed by a physical therapist), compression (a 4-cm gauze bandage from distal hand to armpit and a 6-cm bandage from wrist to armpit were applied for a minimum of 20 hours per day), and exercise (performed twice a day for 15 minutes while wearing compression bandages).
After 7 sessions of treatment, the changes in the left arm circumference were as follows: upper arm, from 22 cm to 19 cm; forearm, from 22.5 cm to 21 cm, respectively. Bioimpedance analysis of the left upper limb showed a decrease in water retention from 1,050 mL to 980 mL, and a decrease in moisture ratio from 0.412% to 0.405%. Surgery was not performed because the lymphedema of this case was not severe enough to have significant functional limitations. Economic hardship forced the patient's return to Russia before further evaluations, including genetic analysis, and long-term rehabilitation could be performed.
We diagnosed a rare congenital lymphatic abnormality, Hennekam syndrome, and were able to demonstrate improvement in the patient's condition after only a brief period of rehabilitation treatment.
Go to :

DISCUSSION
In this case, we suspected Hennekam syndrome based on congenital left-arm edema and typical facial features. Lymphatic malformations of multiple organs were confirmed by CT angiography, lymphatic scan, chest CT, and abdominal US.
The facial features of Hennekam syndrome patients typically include a flattened appearance of the face, a flat and broad nasal bridge, hypertelorism, and epicanthal folds, as was the case for this patient [
3]. Other facial characteristics such as low set and dysplastic ears, smooth philtrum, and dental anomalies were not evident in this case. The patient's facial features were similar to those observed in previous cases of Hennekam syndrome, and it was these facial features that led to the diagnosis.
The developmental abilities of patients with Hennekam syndrome have ranged from almost normal psychomotor development to varying levels of mental retardation [
3]. Application of rehabilitative developmental assessment tools showed slightly delayed psychomotor development. Due to the young age of the patient, 28 months, periodic follow-up of psychomotor development is required.
Intestinal lymphangiectasia can lead to severe protein loss resulting in hypogammaglobulinemia, hypoalbuminemia, lymphopenia, and growth retardation [
6]. It has been reported that growth retardation may be caused by both gastrointestinal protein loss and malabsorption [
6]. Ultrasonographic findings of intestinal lymphangiectasia were found in this patient, but no protein-loss pattern or growth retardation pattern was observed.
The congenital pulmonary lymphangiectasis (CPL) is a rare developmental disorder of the pulmonary lymphatic vessels [
7]. Early death of the affected newborn is usually caused by severe pulmonary hypoplasia [
7]. A chest CT showed pulmonary lymphangiectasis in this patient, which may have been related to the NICU care at the time of birth. Pulmonary-related complications are directly related to survival, so regular follow-up chest X-rays or chest CTs are required.
About 33% of cases of Hennekam syndrome patients have seizures, varying from absences to tonic or atonic attacks [
3]; however, this patient had never experienced a seizure. The patient's brain MRI showed focal increased signal intensity in right periventricular white matter, which implies gliotic change or periventricular leukomalacia.
Treatment will vary according to symptoms [
3]. Some patients may require a high-protein, low-fat diet with medium-chain triglyceride supplementation in the case of protein-losing enteropathy [
8]. The lymphedema may be severely disabling, but surgery [
9] is considered a treatment of last resort. Surgery may improve limb size and restore some function in severe lymphedema that does not respond to conservative treatment.
The significance of this case report is that it is the first paper on rehabilitative evaluation and treatment for Hennekam syndrome. Also, to our knowledge, this is the first Russian case to be reported in Korea. The limitations of this case report are the lack of genetic testing for the patient and the lack of follow-up care of the patient.
In summary, the patient was diagnosed with Hennekam syndrome based on clinical phenotypic features such as lymphangiectasia, lymph edema, and facial anomalies. There is no curative therapy at this time, but rehabilitative treatments including complete decongestive therapy for edema control appeared to be beneficial.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download