Abstract
Objective
To investigate the immediate therapeutic effects of mental singing while walking intervention on gait disturbances in hemiplegic stroke patients.
Methods
Eligible, post-stroke, hemiplegic patients were prospectively enrolled in this study. The inclusion criteria were a diagnosis of hemiplegia due to stroke, and ability to walk more than 10 m with or without gait aids. Each patient underwent structured music therapy sessions comprising 7 consecutive tasks, and were trained to sing in their mind (mental singing) while walking. Before, and after training sessions, gait ability was assessed using the 10-Meter Walk Test (10MWT), the Timed Up and Go test (TUG), gait velocity, cadence and stride length.
Results
Twenty patients were enrolled in the interventions. Following the mental singing while walking intervention, significant improvement was observed in the 10MWT (13.16±7.61 to 12.27±7.58; p=0.002) and the TUG test (19.36±15.37 to 18.42±16.43; p=0.006). Significant improvement was also seen in gait cadence (90.36±29.11 to 95.36±30.2; p<0.001), stride length (90.99±33.4 to 98.17±35.33; p<0.001) and velocity (0.66±0.45 to 0.71±0.47; p<0.002).
Gait disturbance is one of the major challenges encountered in patients diagnosed with hemiplegic stroke. It limits their quality of life, and functional independence. Specifically, abnormal stride length, short or narrow steps, and poor timing of muscle contraction and relaxation in the affected lower limb, interfere with gait speed of stroke survivors [1].
External cues have been reported to promote motor skill learning [2]. Rhythmic auditory stimulation (RAS) has been proposed as an effective therapeutic method to improve gait function, which is rhythmic in itself [3]. Suh et al. [4] suggested that 3 weeks of gait training with RAS significantly improved gait velocity, stride length, cadence, and standing balance in hemiplegic stroke patients. Further, Thaut et al. [5] reported that after 3 weeks of gait training, RAS showed significantly better clinical outcomes in terms of gait parameters, compared with neurodevelopmental therapy/Bobath-based training in early post-stroke patients. RAS had an immediate effect on motor output [6].
In most previous studies, RAS was usually administered by repetitive, rhythmic beats delivered by a metronome or similar device, sometimes contained in music. However, additional equipment or instruction was required to carry out these training methods using external cues. Moreover, RAS may require additional resources for stroke patients who have relatively decreased cognitive reserves and restricted attentional resources that impair multitasking; recognition of the tempo of beats comparing the gait speed with the given beats and adjusting the walking pace [7]. Compared with healthy adults, who can change their gait parameters and rhythmicity unconsciously while walking [8], RAS for stroke survivors can be a cognitively-demanding task requiring multi-step processing.
Satoh and Kuzuhara [9] initially studied the effect of mental singing on gait disturbance in patients with Parkinson disease. This method uses singing as an inner cue while walking, and was found to significantly improve gait dysfunction in patients with Parkinson disease. Mental singing requires cognitive resources for output alone, while external cueing requires cognitive resources for both input and output. Furthermore, mental singing does not require extra devices, and is less restricted by space and time, as the cueing results from patients' singing in their mind. To date, the effect of mental singing while walking has not been investigated in gait disturbance after stroke.
In this study, the therapeutic effects of mental singing while walking were evaluated in hemiplegic stroke patients by measuring their gait and balance. In the absence of previous studies, the aim of the current study is to determine the immediate effects of mental singing while walking on gait disturbance in stroke patients.
Patients were recruited at Soonchunhyang University Bucheon Hospital between July 2016 and October 2016. The inclusion criteria were: (1) diagnosis with hemiplegia after stroke, indicated by magnetic resonance imaging and computed tomography; (2) an onset time of less than 12 months; (3) absence of previous history of stroke; (4) inability to walk more than 10 m with, or without gait aids; and (5) a score of 24 or higher on the Korean version of the Mini-Mental State Examination (MMSE). The exclusion criteria were: (1) severe hearing difficulty and inability to follow instructions; (2) inability to sing a song due to aphasia; (3) a history of neurologic disease other than stroke; (4) orthopedic challenges associated with lower limbs; and (5) uncontrolled medical conditions affecting gait function.
The sample size was calculated using the G*Power version 3.1.9.2 (Heinrich Heine University, Dusseldorf, Germany). Power was set at 0.80, with an alpha 0.05, and effect size 0.68. Assuming an attrition rate of 10%, an estimated total sample size of 20 was needed.
All participants were informed of the study procedures and objectives. The study protocol was approved by the Institutional Review Board of Soonchunhyang University Buchoen Hospital (No. SCHBC2016-03-017).
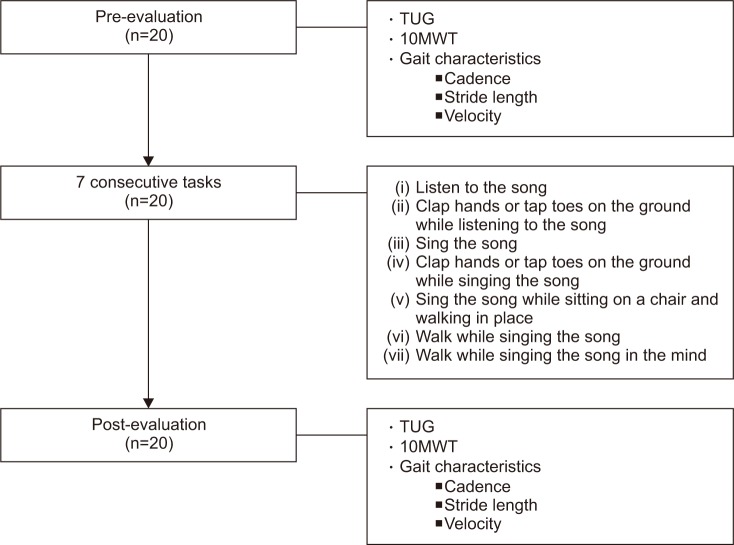
During the mental singing sessions, each patient performed 7 consecutive tasks, previously proposed by Satoh and Kuzuhara [9], using a song played by a smartphone. The song selected for this study was ‘Santokki (a jack rabbit)’, a well-known Korean children's song published in the year 1938. The tasks were as follows: (i) listening to the song; (ii) clapping hands or tapping toes on the ground while listening to the song; (iii) singing; (iv) clapping or tapping while singing; (v) singing while sitting on a chair and walking in place; (vi) walking while singing, and (vii) walking while mentally singing the song.
The main objective of these consecutive tasks was to comfortably prepare for the final task. Therefore, the first 6 tasks were set in order to easily master the last task. During task (vi) and (vii), patients were taught to walk comfortably for 5 m straight ahead on a flat ground and return to the starting position with or without their gait aids, for a total walking distance of 10 m involving 2 turns. Each task was repeated a few times until the participants understood the meaning and goal of the task completely.
To investigate the immediate effects of mental singing on clinical functional outcomes and gait characteristics, patients were evaluated before task (i) (pre-evaluation) and after task (vii) (post-evaluation) (Fig. 1).
The 10-Meter Walk Test (10MWT) and the Timed Up and Go (TUG) tests were used to assess the clinical outcomes of training. The 10MWT has high reliability and excellent predictive validity according to the Barthel Index and instrumental activities of daily living in stroke patients [1011]. The TUG test, which is used to evaluate the risk of falls among post-stroke patients [12], is positively correlated with gait and endurance in chronic stroke patients [13]. It is usually recommended for gait assessment in persons with mild-to-moderate hemiparesis after stroke [10].
Changes in gait were evaluated by cadence, stride length and velocity. These parameters were estimated using a method reported by Hayden et al. [11]; in which an examiner counted steps and measured the time while another examiner evaluated the length of two steps, during the course of a patient's walk along a straight path of 10–15 m.
Each patient was tested by two examiners who were not engaged in this study and instructed to walk at a self-selected brisk walking speed. Four trials were accomplished for each test. The mean values of the last three trials were analyzed, with the first one regarded as a pre-practice trial. All of the patients were provided a 1-minute interval of rest between each trial and a 2-minute rest period between each test.
Statistical Package of Social Science (SPSS) version 14.0K for windows (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The results were expressed as a number on a categorical scale, and as the mean±standard deviation on a continuous scale. The changed values of the outcomes were determined, after the mental singing while walking data were analyzed with the paired t-test or Wilcoxon signed-rank test as appropriate following a Shapiro-Wilk test for normality. The level of statistical significance was set at p<0.05.
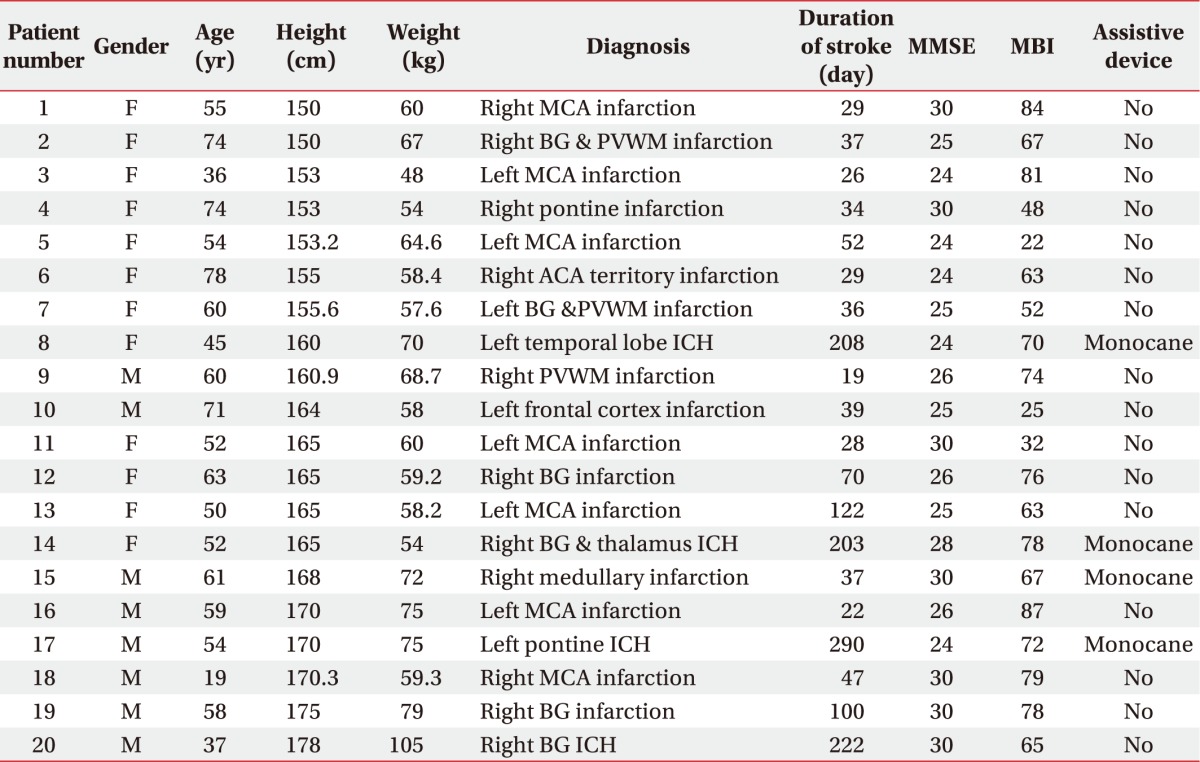
Twenty post-stroke patients completed the entire study. The mean age was 55.6±14.1 years (range, 19–78 years). Of the 20 patients, 12 were female including 11 with a right-sided stroke lesion and 17 with a supratentorial stroke lesion. The mean K-MMSE score was 26.8±2.6 (range, 24–30 scores), the mean duration of stroke was 82.5±81.8 days (range, 19–290 days), and the mean Korean version of Modified Barthel Index score was 64.2±19.1 (range, 22–87 scores). Sixteen patients walked without any assistive devices and the others walked with a single cane. Table 1 shows the patients' demographic and clinical data.
Most participants accomplished each of the seven consecutive tasks, immediately. However, three participants failed to understand all of the tasks at one time. None of the patients required repetition of instructions more than three times. The time required to complete the 7 consecutive tasks was approximately 30 minutes.
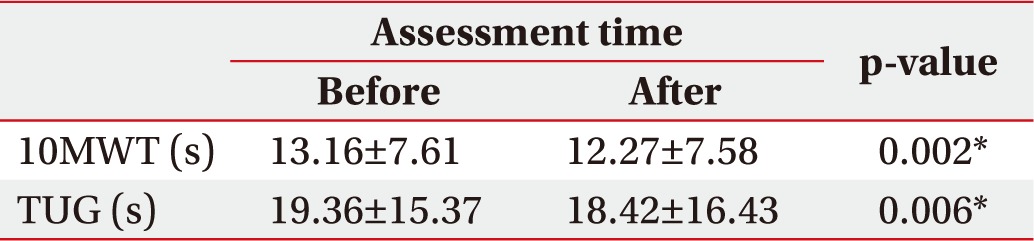
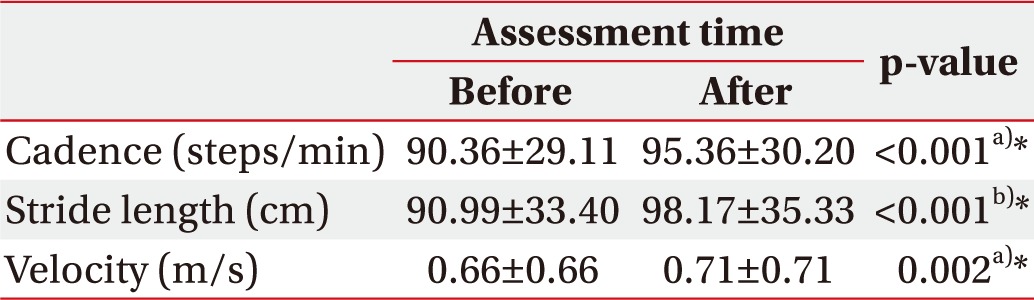
After mental singing while walking, significant improvement was found in the 10MWT (13.16±7.61 to 12.27±7.58; p=0.002), and the TUG test (19.36±15.37 to 18.42±16.43; p=0.006) (Table 2). In terms of gait, we found significant improvement in cadence (90.36±29.11 to 95.36±30.2; p<0.001), stride length (90.99±33.4 to 98.17±35.33; p<0.001), and velocity (0.66±0.45 to 0.71±0.47; p<0.002) (Table 3).
This study demonstrated the possibility that mental singing while walking instantly improves, not only clinical functional outcomes, but also the gait parameters of hemiplegic stroke patients.
The song selected for this study was ‘Santokki (a jack rabbit)’, a well-known Korean children's song. This song was selected for two reasons. First, all the participants were very familiar with the song. Leow et al. [14] suggested that familiarity with the music increased walking speed when using rhythmic auditory cueing. Cognitive demands of synchronizing were reduced with beat structure familiarity leading to better synchronization and a faster less variable gait. Second, this song has a two-four beat, and is generally played at a medium-tempo of 90–120 beats per minute (bpm). Silva et al. [15] investigated the effect of music tempo on the rating of perceived exertion (RPE), during self-selected paced walking. In their study, the participants underwent three 30-minute walking sessions in a counterbalanced order: fast-tempo music (140–150 bpm), medium-tempo music (115–120 bpm), and no-music control. They found that the RPE was lower with the medium-tempo music compared with the control. However, the RPE showed no differences between the fast-tempo and the control conditions. These results suggest that the medium-tempo music apparently alleviated the physiological feedback derived from walking, while the fast-tempo music may decrease motivation leading to loss of interest in the activity [16]. Therefore, we considered that this song might be suitable for mental singing while walking.
RAS is known to activate motor neurons at the spinal level, and in the brainstem [17]. Suteerawattananon et al. [18] reported that RAS increased the excitability of spinal motor neurons via the reticulospinal tract, and thus shortened the muscle reaction time in response to motor commands, contributing to the improvement in gait speed. Rossignol and Jones [17] investigated the audio-spinal influences by using an H-reflex technique and non-startling sounds. They reported that sound potentiated the H-reflex amplitude during hopping to a simplified musical rhythm. This observation suggested that during synchronized stereotyped movements to repetitive auditory stimuli, the descending motor events were entrained to the auditory rhythmic signal to leverage the effect of potential audio-spinal facilitation. The cerebellum and thalamus as well as the brainstem, whichplay a key role in timing mechanisms, are also affected by RAS, which apparently compensates for the motor timing dysfunction leading to enhanced gait parameters [19]. Shin et al. [20], reported that gait training with RAS improves both kinematic and temporospatial patterns in hemiplegic stroke patients. Cha et al. [21] investigated the immediate effects of gait training with RAS on hemiplegic stroke patients. The authors indicated that RAS, which was delivered at a 10%–20% faster tempo compared with patients' baseline walking speed, showed the potential for instant improvement in walking performance.
Most previous studies investigating the effect of gait training with RAS utilized external cueing for auditory stimulation. Although, Satoh and Kuzuhara [9] used inner cueing as their intervention for Parkinson disease limited evidence is available to confirm the role of inner cueing on the ambulatory abilities of stroke patients. Consistent with previous study results involving RAS, the present study demonstrated that mental singing while walking instantly improved gait parameters and clinical functional outcomes in hemiplegic stroke patients. Compared with other methods reported previously the mental singing while walking intervention with inner cueing provides benefits for stroke patients.
First, it can be utilized anytime and anywhere. The external cueing can be provided by music or repeated sounds from a metronome, which was also used as a therapy in previous studies, and is more suitable for a clinical setting than a daily living situation. The method requires a musical device or a tool and occasional guidance of a therapist. By contrast, mental singing uses a mental song as a cue and therefore, can be used to improve gait disturbance under ordinary circumstances. Patients can master the sequences without the need for a therapist.
Second, the mental singing while walking intervention requires fewer cognitive resources than externally cued methods. When performing RAS cognitive capacity represents an important factor because walking with auditory cueing requires specific cognitive resources [22]. In patients with Alzheimer disease, it was reported that both musical and metronome cueing disturbed patients' gait performance in the absence of a movement disorder [23]. Attention, which is essential for learning motor skills, is usually impaired following stroke, and its deficiency reduces physical functioning [2425]. Therefore, previous methods utilizing external cueing might demand excessive attention in stroke patients. The cognitive demands of this method for both input and output, require patients to control their walking in tandem with external cues. However, mental singing only requires cognitive processing for output, which is less demanding on patients' cognitive resources. It requires less attention and is much simpler and easier to perform than previous methods. The patients are able to master this method with about 30 minutes of practice.
Even though the exact mechanisms for improving gait by mental singing remain unclear, we hypothesize that the mechanism was similar to that of RAS [1718]. Other factors include emotional engagement and motivation. Singing is an emotionally engaging process more than listening to music or the sound of a metronome and therefore, patients in this study might be highly motivated to participate actively in the training sessions. Incorporating affective and motivational factors into any intervention without overlooking the relationship between the pure motor network and emotional effects may hold the key to understanding the underlying mechanisms [26].
This feasibility study has several limitations. First, the small sample size makes it difficult to generalize our results to hemiplegic stroke patients. Second, since we only confirmed the immediate effects of the intervention, no short- and long-term effects were evaluated. Third, it is possible that the improved gait in post-evaluation may be attributed to gait repetition during pre-evaluation—tasks (vi) and (vii)—rather than the immediate effect of mental singing. Fourth, gait parameters were not evaluated kinematically using a motion analysis system. Finally, we did not distinguish patients based on their site of damage. However, the auditory rhythm is processed bilaterally and no difference in performance was observed between patients with left- and right-hemiparesis in previous studies [3].
In conclusion, mental singing while walking demonstrated immediate positive effects in improving the functional outcomes and gait parameters of patients diagnosed with hemiplegic stroke. To the best of our knowledge, this is the first study which investigated the effects of mental singing while walking in hemiplegic stroke. This simple and feasible method may be used as an alternate gait training method for hemiplegic stroke patients in both clinical and non-clinical settings. In order to clarify the effects of this novel method, additional studies with a control group and long-term follow-up are required to address the limitations of this study.
References
1. Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain. 1998; 121(Pt 3):515–526. PMID: 9549527.

2. Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Med Educ. 2010; 44:75–84. PMID: 20078758.

3. Thaut MH, McIntosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997; 151:207–212. PMID: 9349677.

4. Suh JH, Han SJ, Jeon SY, Kim HJ, Lee JE, Yoon TS, et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation. 2014; 34:193–199. PMID: 24284453.

5. Thaut MH, Leins AK, Rice RR, Argstatter H, Kenyon GP, McIntosh GC, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007; 21:455–459. PMID: 17426347.
6. Thaut MH, Kenyon GP. Rapid motor adaptations to subliminal frequency shifts during syncopated rhythmic sensorimotor synchronization. Hum Mov Sci. 2003; 22:321–338. PMID: 12967761.

7. Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002; 50:1572–1576. PMID: 12383157.

8. Molinari M, Leggio MG, De Martin M, Cerasa A, Thaut M. Neurobiology of rhythmic motor entrainment. Ann N Y Acad Sci. 2003; 999:313–321. PMID: 14681155.

9. Satoh M, Kuzuhara S. Training in mental singing while walking improves gait disturbance in Parkinson's disease patients. Eur Neurol. 2008; 60:237–243. PMID: 18756088.
10. Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005; 37:75–82. PMID: 15788341.
11. Hayden R, Clair AA, Johnson G, Otto D. The effect of rhythmic auditory stimulation (RAS) on physical therapy outcomes for patients in gait training following stroke: a feasibility study. Int J Neurosci. 2009; 119:2183–2195. PMID: 19916847.

12. Andersson AG, Kamwendo K, Seiger A, Appelros P. How to identify potential fallers in a stroke unit: validity indexes of 4 test methods. J Rehabil Med. 2006; 38:186–191. PMID: 16702086.

13. Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005; 86:1641–1647. PMID: 16084820.
14. Leow LA, Rinchon C, Grahn J. Familiarity with music increases walking speed in rhythmic auditory cuing. Ann N Y Acad Sci. 2015; 1337:53–61. PMID: 25773617.

15. Silva AC, Dos Santos Ferreira S, Alves RC, Follador L, DA Silva SG. Effect of music tempo on attentional focus and perceived exertion during self-selected paced walking. Int J Exerc Sci. 2016; 9:536–544. PMID: 27990220.
16. Karageorghis C, Jones L, Stuart DP. Psychological effects of music tempi during exercise. Int J Sports Med. 2008; 29:613–619. PMID: 18050063.

17. Rossignol S, Jones GM. Audio-spinal influence in man studied by the H-reflex and its possible role on rhythmic movements synchronized to sound. Electroencephalogr Clin Neurophysiol. 1976; 41:83–92. PMID: 58771.

18. Suteerawattananon M, Morris GS, Etnyre BR, Jankovic J, Protas EJ. Effects of visual and auditory cues on gait in individuals with Parkinson's disease. J Neurol Sci. 2004; 219:63–69. PMID: 15050439.

19. Kobinata N, Ueno M, Imanishi Y, Yoshikawa H. Immediate effects of rhythmic auditory stimulation on gait in stroke patients in relation to the lesion site. J Phys Ther Sci. 2016; 28:2441–2444. PMID: 27799666.

20. Shin YK, Chong HJ, Kim SJ, Cho SR. Effect of rhythmic auditory stimulation on hemiplegic gait patterns. Yonsei Med J. 2015; 56:1703–1713. PMID: 26446657.

21. Cha Y, Kim Y, Chung Y. Immediate effects of rhythmic auditory stimulation with tempo changes on gait in stroke patients. J Phys Ther Sci. 2014; 26:479–482. PMID: 24764615.

22. Ijmker T, Lamoth CJ. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012; 35:126–130. PMID: 21964053.

23. Wittwer JE, Webster KE, Hill K. Effect of rhythmic auditory cueing on gait in people with Alzheimer disease. Arch Phys Med Rehabil. 2013; 94:718–724. PMID: 23159787.

24. Hochstenbach J, Mulder T, van Limbeek J, Donders R, Schoonderwaldt H. Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol. 1998; 20:503–517. PMID: 9892054.

25. Hyndman D, Pickering RM, Ashburn A. The influence of attention deficits on functional recovery post stroke during the first 12 months after discharge from hospital. J Neurol Neurosurg Psychiatry. 2008; 79:656–663. PMID: 17872979.

26. Schaefer RS. Auditory rhythmic cueing in movement rehabilitation: findings and possible mechanisms. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130402. PMID: 25385780.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download