Abstract
Objective
To examine the long-term effects of the low-frequency repetitive transcranial magnetic stimulation (LFrTMS) combined with task-specific training on paretic hand function following subacute stroke.
Methods
Sixteen participants were randomly selected and grouped into two: the experimental group (real LFrTMS) and the control group (sham LF-rTMS). All the 16 participants were then taken through a 1-hour taskspecific training of the paretic hand. The corticospinal excitability (motor evoke potential [MEP] amplitude) of the non-lesioned hemisphere, and the paretic hand performance (Wolf Motor Function Test total movement time [WMFT-TMT]) were evaluated at baseline, after the LF-rTMS, immediately after task-specific training, 1 and 2 weeks after the training.
Results
Groups comparisons showed a significant difference in the MEP after LF-rTMS and after the training. Compared to the baseline, the MEP of the experimental group significantly decreased after LF-rTMS and after the training and that effect was maintained for 2 weeks. Group comparisons showed significant difference in WMFT-TMT after the training. Only in the experimental group, the WMFT-TMT of the can lifting item significantly reduced compared to the baseline and the effect was sustained for 2 weeks.
Limited function of the paretic upper extremity (UE) is one of the most disabling consequences of stroke [1]. Approximately 55% of stroke survivors had no UE movement following initial rehabilitation [2] which made them more of a burden to their relatives and society as a whole. After stroke, functional recovery is associated with interhemispheric communication. Studies using transcranial magnetic stimulation (TMS) reported that the nonlesioned hemisphere had more corticospinal excitability than the lesioned hemisphere [3-5]. The suppression of the recovery in the lesioned hemisphere leads to persistent functional deficits [3,4,6-9]. Therefore, rebalancing the corticospinal excitability of both hemispheres may support restoration of the function.
The existing evidence supports that the application of low-frequency repetitive TMS (LF-rTMS) could reduce the corticospinal excitability (CE) of the non-lesioned hemisphere (e.g., see [8-13]). TMS is a noninvasive technique that modulates excitability of the cerebral cortex [3,6]. Several studies confirmed that reduction of CE by LF-rTMS leads to the improvement of the paretic hand function [8-14]. Among those studies, only a few study added motor training after a single session of LF-rTMS [11,13,14]. Takeuchi et al. [11] analyzed the combined effects of LF-rTMS with 15-minutes pinch training in chronic stroke. They reported improvement in acceleration of pinching and pinch force in the rTMS group as compared to the pinch training alone group. Vongvaivanichakul et al. [13] examined the effect of LF-rTMS on outcomes following reach-to-grasp (RTG) training in chronic stroke. They reported improvement in movement time on the Wolf Motor Function Test and the time of RTG actions in the experimental group, as compared to the control group. Moreover, the results of Takeuchi’s study [11] showed that the improvement of paretic hand maintained for one week. These findings were possible due to the adjunctive treatment protocol [11]. Of particular interest, there was a review paper that reported the use of non-invasive brain stimulation to augment motor training-induced plasticity [15]. Therefore, an adjunctive protocol is needed to promote the recovery of motor performance. However, the adjunctive protocol used in both studies included a simple pinch task, not a real-world activity. Therefore, it is necessary to identify a type of motor training that involves a daily activity, thereby inducing neural plasticity and long-term behavioral changes.
The Accelerated Skill Acquisition Program (ASAP) and constraint-induced movement therapy (CIMT) are types of task-specific training [16-18]. The ASAP is an innovative, yet fully-defined, evidence-based and theoretically defensible therapy program. It is a hybrid combination of CIMT [19] and skill-based/impairment-mitigating motor learning training with embedded motivational enhancements [16,20,21]. The ASAP provides restoration of the paretic limb by employing tasks that involve real training situations, thereby reducing motor impairments and compensatory strategies [16,21]. CIMT focuses on restraining the movement of the non-paretic hand to prevent adaptive behavior, thus overcoming a phenomenon of ‘learned non-use’ [16-18]. Inhibition of the non-paretic hand is related to the mechanism by which LF-rTMS reduces hyperexcitability of the non-lesioned hemisphere. The aforementioned studies [11,13] reported the combined effects of LF-rTMS and motor training in chronic stroke, its effectiveness with subacute stroke (1–6 months post onset) is however still unclear. We hypothesized that if the adjunctive intervention is assigned to early-onset individuals and the motor training is a task-specific one, the improvement would last more than a week. We based the hypothesis on the principle of experience-induced plasticity, i.e., time matters and task specificity [22]. We recently reported part of the results on the immediate effect of LF-rTMS to enhance task-specific training [14]. Therefore, the objectives of this study were two-fold: (1) to examine the long-term effects of a single session of LF-rTMS combined with task-specific training using the ASAP principles on the paretic hand performance in individuals with subacute stroke, and (2) to determine the relationship between changes in corticospinal excitability of the non-lesioned hemisphere and the improvement of paretic hand function.
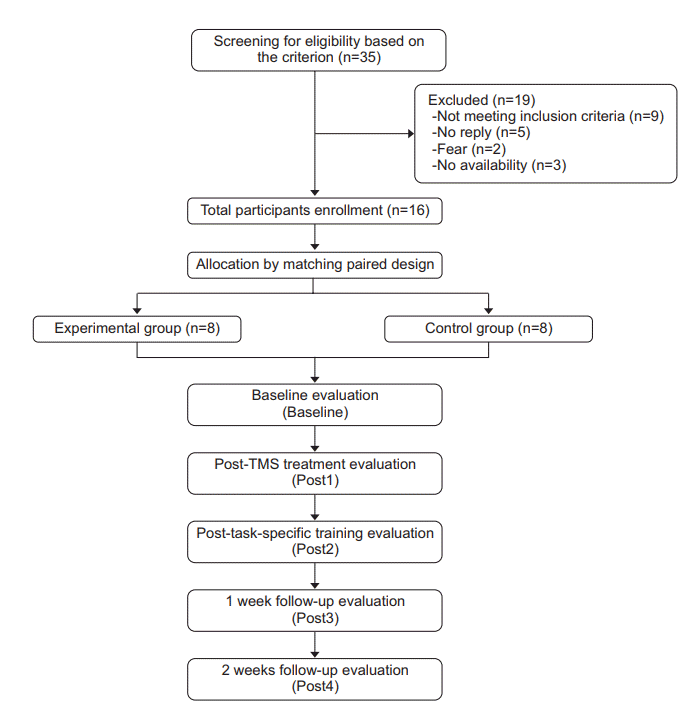
Individuals, from the physical therapy centers of the Faculty of Physical Therapy, Mahidol University, Siriraj Hospital, and Golden Jubilee Medical Center, Mahidol University, with a first-time subacute stroke were screened for eligibility (Fig. 1). The inclusion criteria were as follows: (1) subcortical stroke within 1–6 months as verified by the CT/MRI findings; (2) must be between the age of 20–79 years; (3) right handedness based on the Edinburgh Handedness Inventory; (4) must have mild to moderate impairment of an UE based on the Fugl-Meyer Assessment; (5) must be able to follow simple commands as evaluated by the 2002 Thai version of the Mini Mental State Examination; (6) must be able to perform mass extension of finger; (7) must be able to sit independently for more than 1 hour; (8) must have normal/corrected hearing and vision; and (9) must have no aphasia. The exclusion criteria were as follows: (1) contraindication of TMS, e.g., seizures, as confirmed by a TMS screening questionnaire; (2) other neurological/musculoskeletal problems affecting the UE and interfering with task achievement; (3) lesions in other brain areas, e.g., the brainstem; and (4) receipt of task-specific training during the study period. This study was approved by the Mahidol University Institutional Review Board (No. MU-IRB 2012/064.0304) and the Siriraj Hospital Institutional Review Board (No. SIRB SI134/2013). All participants were given a written informed consent form, and they signed the consent form prior to the enrollment.
This study used a double-blinded matched-pair experimental design. Participants were randomly grouped into two, the experimental and control groups, using a convenience sampling method (Fig. 1). They were matched according to their extent of UE impairment, paretic side and age range (±5 years). The experimental group received real rTMS with task-specific training, whereas the control group received sham rTMS with task-specific training. For each participant, corticospinal excitability and the performance of the paretic hand were evaluated 5 times: at baseline (Baseline); immediately after rTMS (Post1); immediately after task-specific training (Post2); 1 week after task-specific training (Post3); and 2 weeks after task-specific training (Post4). The evaluations were performed by a blinded assessor. During the follow-up period, the participants were asked to keep a daily record of their UE and to report these results to the assessor, the research assistant assigned for group allocation. The coinvestigator assessed all participants in each test.
The LF-rTMS was applied over M1 of the non-lesioned hemisphere at the hotspot of the extensor digitorum (ED) muscle (1 Hz, 90% of resting motor threshold [rMT], 1,200 pulses). The stimulation was delivered through the figure-eight air-cooled coil with a Magstim Rapid2 stimulator (Magstim Co., Whitland, UK). However, the control group received sham stimulation applied using the same coil placement and same TMS parameter as the experimental group except that the coil was tilted 90° [11,23]. We performed the stimulation protocol under the safety guidelines of rTMS application [24].
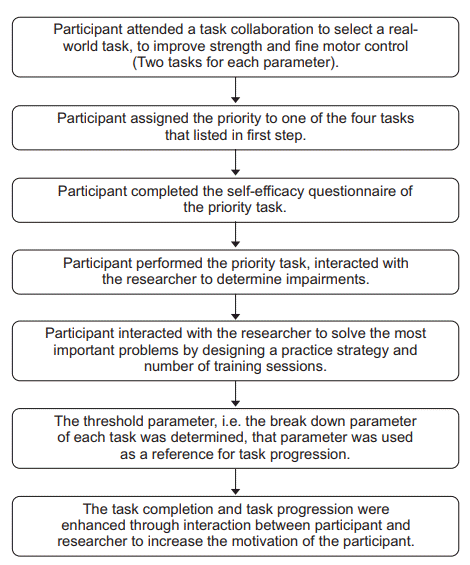
The task-specific training was modified from the basic procedures for CIMT and the ASAP. The procedure for CIMT was the restraint of the non-paretic hand by a mitt during the training. The conceptual framework of the ASAP consists of three domains: task specificity, capacity, and motivation [16]. The ASAP process consisted of task collaboration, priority task selection, self-efficacy evaluation, task analysis, threshold finding, and task progression. The protocol is illustrated in detail in Fig. 2. The specific tasks chosen by the participants were as follows; grasping a glass with or without a handle, manipulation of a pen during writing, manipulation of a spoon or fork during eating, and transporting a pot containing five cans of rice during cooking. Each participant underwent 1 hour of task-specific training after the TMS intervention.
The main outcome measurements were the corticospinal excitability of the non-lesioned hemisphere, measured by the motor evoked potential (MEP) amplitude of ED M1, and the performance of the paretic hand, measured by total movement time on the Wolf Motor Function Test (WMFT-TMT).
The corticospinal excitability of the non-lesioned hemisphere, as represented by the peak-to-peak amplitude of MEPs, was measured using single-pulse TMS with a figure-eight coil. The hotspot of ED M1 was marked on the scalp and on the neuro-navigation system to ensure precision. Determining corticospinal excitability also required electromyographic (Medelec Synergy; VIASYS HealthCare Inc., Surrey, UK) recording from silver/silver chloride electrodes positioned in a belly-tendon montage on the skin overlying the ED muscle. First, we measured the rMT of the non-lesioned hemisphere. The rMT refers to the lowest intensity that induces MEPs of 50-μV peakto-peak amplitude in the target muscle in 50% of the trials applying TMS to the ‘optimal site’ [25]. The peak-topeak MEP amplitude was then determined for ten trials at 120% of rMT.
The performance of the paretic hand was measured based on the hand-related items of the WMFT (i.e., can lifting, pencil lifting, picking up a paper clip, stacking checkers, turning a key in a lock, and folding a towel). The grip strength of the paretic hand was also evaluated. During the test, participants comfortably seated in a chair and were instructed to perform the functional test. We demonstrated how to perform each task twice, then the participants performed the task. The participants had a maximum of 2 minutes to complete the task. The movement time on each task was recorded using a stopwatch.
We calculated sample size based on the study using Vongvaivanichakul et al. [13] to detect an effect size of (μ1–μ2), n=2σp2(Zα/2+Zβ)2/(μ1–μ2)2 at confidence level (α) 0.05 and 80% power (1–β) where σp2 represents pooled variance. With 20% dropout, the sample size per group (n) was found to be 8. The Shapiro-Wilk test was used to examine the distribution of the data. The demographic and baseline clinical characteristics were analyzed using descriptive statistics and were comparison between the two groups done using an independentsamples t-test. Also, two-factor ANOVA (Group×Time) with repeated measurements (mixed model) was used to compare behavioral outcomes (average total movement time [TMT], movement time [MT] for each item, and grip strength) and corticospinal excitability (MEP amplitude in the non-lesioned hemisphere) in each testing session between the experimental and control groups and also within the groups. Multiple comparisons were done using the Bonferroni method. The Bonferroni test was used to analyze the behavioral outcomes and corticospinal excitability differences at each testing session between the experimental and control groups. To further examine if there was a relationship between changes in corticospinal excitability of the non-lesioned hemisphere and changes in hand performance following the intervention, we analyzed the Pearson product (parametric) or Spearman (non-parametric) correlation (depending on normality). Significance level was set at p<0.05. SPSS statistical software version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
We screened 35 individuals that had previously experienced a stroke. Sixteen of these individuals met the inclusion criteria, and randomly grouped into two. No participants reported any adverse side effects, and all participants completed the study. The lesion locations in the 16 participants are shown in Table 1. All of the participants had ischemic stroke, except one participant in the experimental group (participant #006). Additionally, there were 4 right hemispheric stroke in the experimental (real TMS) groups and 5 right hemispheric stroke in the control (sham TMS) group. However, the one nonmatched pair of lesion side between the two groups did not impact their average paretic hand impairment (Fugl-Meyer score) as shown in Table 2. There were no differences in age, level of upper extremity impairment, time since stroke, or cognitive function between the experimental and the control groups (Table 2).
The main effect of time (F(3, 39)=1.096, p=0.362), main effect of group (F(1, 13)=2.911, p=0.112), and interaction effect of time by group (F(3, 39)=1.009, p=0.399) were all insignificant. Although there was no interaction of time by group, we aimed to examine between-group comparisons at each time point. For the non-lesioned hemisphere, there were significant group differences in percentage change from baseline of the MEP amplitude of the non-lesioned hemisphere immediately after LFrTMS (post1; p=0.043, ES=0.60) and immediately after task-specific training (post2; p=0.047, ES=0.59). Based on within-group comparisons, there was no change in the MEP amplitude in the control group over time. On the contrary, the experimental group showed a significant reduction in the MEP amplitude immediately after the real LF-rTMS compared to after the baseline (p<0.001). Furthermore, the reduction in percentage change amplitude MEP was maintained after task-specific training (post2) compared to after the baseline (p=0.003) and at 2 weeks after the training (post4) (Table 3).
As for the WMFT-TMT, there was a significant main effect of group (F(1, 13)=5.142, p=0.041, ES=0.55). However, there were no effect of time (F(3, 39)=2.309, p=0.091) or time×group interaction (F(3, 39)=0.712, p=0551). Post hoc analysis revealed a significant difference in TMT of the paretic hand at post2 between the two groups (p=0.023, ES=0.62). On the contrary, there was insignificant difference in the average TMT of the paretic hand at post1 (p=0.082), post3 (p=0.088), or post4 (p=0.075) between the two groups (Table 3).
When each item was analyzed, the movement time of the paretic hand significantly changed within the experimental group over time. This difference was observed only for the can lifting item at post2 (p=0.03, ES=0.62), post3 (p=0.009, ES=0.66), and post4 (p=0.021, ES=0.63) compared to the baseline. On the contrary, the control group showed insignificant differences in the movement time between different time points. Additionally, this pattern of results was not observed for other items of the WMFT.
Based on grip strength, there was a significant main effect of time (p=0.014, ES=0.60). Additionally, there was a significant increase in grip strength at post4 compared to that at post1 in the experimental group but not in the control group (Table 3).
The correlation between changes in corticospinal excitability and the changes in the performance time of the can-lifting item was insignificant (p>0.05). However, the decreased corticospinal excitability of the non-lesioned hemisphere moderately correlated with a decreased performance time of the can-lifting at baseline-post1, baseline-post2 and baseline-post3 only in the experimental group (Table 4).
This study examined the effects of a single session of LF-rTMS combined with task-specific training using the principles of ASAP and CIMT on paretic hand performance following subacute stroke. There was a reduction in MEP amplitude of the non-lesioned hemisphere by the LF-rTMS. This study extended the findings of the studies of Takeuchi [11] and Vongvaivanichakul et al. [13] by evaluating the corticospinal excitability of the nonlesioned hemisphere after task-specific training following subacute stroke. The results of this study showed that the reduction in MEP amplitude induced by the real LFrTMS was maintained immediately after task-specific training. Importantly, the improvement in paretic hand performance after task-specific training was enhanced by LF-rTMS and the effects of conjunctive intervention on the hand function persisted for at least 2 weeks.
The possible mechanisms underlying neural plasticity and improvement of the hand performance by the combination of LF-rTMS and task-specific training are explained in this section. One potential mechanism underlying this effect is that the downregulation of excitability of the non-lesioned hemisphere following LF-rTMS enhances the restoration of balanced interhemispheric inhibition (IHI) [6,8,10,26]. There is evidence that interhemispheric interactions are modulated by the kinematics of the movement [27]. It is possible that downregulation of the non-lesioned M1 decreased the IHI to the ED representational area, thereby improving the hand functions. LF-rTMS leads to the release of the gammabutyric acid (GABAA), which is related to long-term depression (LTD). This process results in the modulation of corticospinal excitability within the human cortex via an inhibitory circuit [20,28]. Similarly, motor training can promote neural plasticity [29-31]. A recent meta-analysis brought about the positive impact of motor training on upper extremity functions. In the same analogy with TMS, task specific training could induce the modulation of NMDA activation receptor and GABAergic inhibition. Applying LF-rTMS before task-specific training might be the prime functional networks for the training [23,32-34]. Therefore, one possible mechanism is that their combination might maximize the effect of each other [15]. Additionally, preventing movement of the non-paretic hand during task-specific training may have contributed to decrease excitability of the non-lesioned hemisphere. This have may enhanced the effect of LF-rTMS with the similar mechanism. These findings are consistent with the results of previous studies [16-18].
The difference in the average MEP amplitude of the non-lesioned hemisphere was insignificant 1 week and 2 weeks after training between the two groups. The MEP amplitude in the experimental group remained decreased after 1 week and 2 weeks of training, whereas, there was no change in the mean MEP amplitude in the control group. These findings are different from the results of the study by Takeuchi et al. [11], who reported that the percentage change in average MEP amplitude of the non-lesioned hemisphere ultimately returned to the baseline level. The simple pinch task used in their study may not have been sufficient to exert a long-term effect.
In addition to corticospinal excitability, hand performance was another variable to reflect the behavioral outcomes of post-stroke rehabilitation. We examined the combined effects of LF-rTMS and task-specific training on the function of the paretic hand using the WMFT. Task-specific training was conducted after a single session of LF-rTMS in both groups. The results showed that compared to the control group, the experimental group showed a greater decrease in TMT of the paretic hand relative to baseline immediately after receiving the real LF-rTMS. The TMT of the experimental group was further decreased after training. Thus, these findings indicate that the benefit of task-specific training was augmented by LF-rTMS. Our protocol was similar to that used in the studies by Takeuchi et al. [11] and Vongvaivanichakul et al. [13]. However, we further extended the protocol of motor training employed by these two studies to more daily functional tasks, i.e., reaching and grasping. This study demonstrated the beneficial combined effect of LFrTMS and task-specific training resulting in the sustained decrease in TMT for at least 2 weeks.
This study’s results are different from those of the study by Takeuchi et al. [11] because their improvements in motor performance lasted for 1 week while ours were longer. Thus, it is essential to combine task-specific training with LF-rTMS. In 2013, Higgins et al. [35] applied 1,200 pulses of LF-rTMS and 90 minutes of task-specific training based on daily activities, 2 sessions/week for 4 weeks. Their results revealed insignificant improvements in WMFT time or grip strength between or within the two groups after the intervention or at the 4-week follow-up visit. The lack of significant improvement may have been due to the small sample size used in that study (n=9). Subsequently, in 2014, Rose et al. [26] applied LF-rTMS combined with functional task practice to chronic stroke patients. All participants received 16 sessions of either active LF-rTMS or sham LF-rTMS followed by 1-hour functional task training of the paretic hand. The results showed insignificant changes in WMFT time or grip force either from baseline to after the intervention or from after the intervention to the 1-month follow-up visit between the two groups. These results are consistent with the study by Higgins et al. [35], although Rose et al. [26] used a larger sample size. Thus, these unexpected findings were possibly due to timing from stroke onset. Both studies [26,35] recruited participants with chronic stroke. Perhaps the combined effects of TMS and training were not sufficiently robust to induce an improvement in paretic hand function following chronic stroke. In contrast to those studies, this study investigated participants with subacute stroke. Accordingly, our findings suggested that the combined effects of LF-rTMS and task-specific training should be assigned to early-onset stroke patients, supporting the principle of experience-induced plasticity, i.e., ‘the earlier the better’ [22]. Although Kakuda et al. [27] reported improvement of UE function in chronic stroke after the combination of rTMS and occupational therapy, the efficacy of the intervention should be confirmed with the control group included. Therefore, this study is the only controlled study that demonstrated the persisted effects of LF-rTMS to augment task-specific training-induced plasticity following subacute stroke.
Regarding hand performance, the movement time on the WMFT included six items. The results of this study showed an insignificant difference in TMT between or within the two groups except for the can lifting item of the experimental group, whose results showed a significant difference in the MT of the paretic hand immediately, 1 and 2 weeks after task-specific training as compared to the baseline. On the contrary, the control group insignificant differences in the MT. Therefore, these findings are possibly due to the similarity of the can lifting task to the task-specific training. The postulation is supported by the analysis of each participant such that most of the participants selected grasping a glass without a handle as their training tasks. Another possible explanation for our result is the target of TMS stimulation, i.e., the wrist extensor muscle, is the essential mediator of lifting a can, so this stimulation may resolve the impairment in the performance of the can lifting task.
Regarding grip strength, there were insignificant differences between the two groups at each testing time. However, there was a significant improvement from immediately after LF-rTMS to 2 weeks after training in the real rTMS group but not in the sham TMS group. These findings indicate that the application of LF-rTMS alone or immediate effects of LF-rTMS combined with task-specific training was insufficient to improve the grip strength of the paretic hand. In the long run, the improvement of the paretic hand in the real rTMS group may have been induced by the combined effects of LF-rTMS, task-specific training, and daily activities. Participants were motivated to build up the confidence and self-management which might have encouraged them to use their hand in their daily activities [21]. The enhanced effect through the mesocortical pathway motivated them to continuously use the affected hand thereby improved grip strength at the retention test of week 2 after the training. During the follow-up period, both groups performed daily activities and received physical therapy. However, the effects of daily activities and physical therapy alone were insufficient to change the grip strength as shown in the sham group.
In conclusion, this study established that the effects of a single session of LF-rTMS on the non-lesioned hemisphere combined with task-specific training led to the paretic hand improvement following subacute stroke. This improvement may be as a result of maximizing each individual effects thereby induced the long-term effects persisted for at least 2 weeks. This study suggests that combining the protocol is a useful intervention for individuals with subacute stroke who has mild to moderate impairment.
ACKNOWLEDGMENTS
This study is supported by Faculty of Physical Therapy and Faculty of Graduate Studies, Mahidol University, Thailand.
REFERENCES
1. American Stroke Association. Impact of stroke (stroke statistics) [Internet]. Dallas: American Stroke Association;2016. [cited 2018 Nov 15]. Available from: http://www.strokeassociation.org/STROKEORG/AboutStroke/Impact-of-Stroke-Stroke-statistics_UCM_310728_Article.jsp#.W_dlSOgzaUl.
2. Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008; 22:706–14.

3. Harris-Love ML, Cohen LG. Noninvasive cortical stimulation in neurorehabilitation: a review. Arch Phys Med Rehabil. 2006; 87(12 Suppl 2):S84–93.

4. Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006; 19:21–33.

5. Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002; 125(Pt 8):1896–907.

6. Park E, Kim YH, Chang WH, Kwon TG, Shin YI. Interhemispheric modulation of dual-mode, noninvasive brain stimulation on motor function. Ann Rehabil Med. 2014; 38:297–303.

7. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004; 55:400–9.

8. Bütefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008; 22:4–21.

9. Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005; 36:2681–6.

10. Dafotakis M, Grefkes C, Wang L, Fink GR, Nowak DA. The effects of 1 Hz rTMS over the hand area of M1 on movement kinematics of the ipsilateral hand. J Neural Transm (Vienna). 2008; 115:1269–74.
11. Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008; 40:298–303.

12. Takeuchi N, Toshima M, Chuma T, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation of the unaffected hemisphere in a patient who was forced to use the affected hand. Am J Phys Med Rehabil. 2008; 87:74–7.

13. Vongvaivanichakul P, Tretriluxana J, Bovonsunthonchai S, Pakaprot N, Laksanakorn W. Reach-to-grasp training in individuals with chronic stroke augmented by low-frequency repetitive transcranial magnetic stimulation. J Med Assoc Thai. 2014; 97 Suppl 7:S45–9.
14. Thanakamchokchai J, Tretriluxana J, Jalayondeja C, Pakaprot N. Immediate effects of low-frequency repetitive transcranial magnetic stimulation to augment task-specific training in sub-acute stroke. Asia Pac J Sci Technol. 2015; 20:105–19.
15. Bolognini N, Pascual-Leone A, Fregni F. Using noninvasive brain stimulation to augment motor traininginduced plasticity. J Neuroeng Rehabil. 2009; 6:8.

16. Winstein CJ, Wolf SL. Task-oriented training to promote upper extremity recovery. In : Stein J, Harvey RL, Macko RF, Winstein CJ, Zorowitz RD, editors. Stroke recovery and rehabilitation. New York: Demos Medical Publishing;2009. p. 267–90.
18. Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006; 19:41–7.

19. Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007; 36 Suppl 2:T164–73.

20. Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006; 5:708–12.

21. Tretriluxana J, Runnarong N, Tretriluxana S, Prayoonwiwat N, Vachalathiti R, Winstein C. Feasibility investigation of the Accelerated Skill Acquisition Program (ASAP): insights into reach-to-grasp coordination of individuals with postacute stroke. Top Stroke Rehabil. 2013; 20:151–60.

22. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008; 51:S225–39.

23. Conforto AB, Cohen LG. Transcranial magnetic stimulation and brain plasticity. In : Hallett M, Chokroverty S, editors. Magnetic stimulation in clinical neurophysiology. 2nd ed. Philadelphia: Elsevier;2005. p. 143–54.
24. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998; 108:1–16.

25. Maeda F, Pascual-Leone A. Transcranial magnetic stimulation: studying motor neurophysiology of psychiatric disorders. Psychopharmacology (Berl). 2003; 168:359–76.

26. Rose DK, Patten C, McGuirk TE, Lu X, Triggs WJ. Does inhibitory repetitive transcranial magnetic stimulation augment functional task practice to improve arm recovery in chronic stroke? Stroke Res Treat. 2014; 2014:305236.

27. Kakuda W, Abo M, Shimizu M, Sasanuma J, Okamoto T, Yokoi A, et al. A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J Neuroeng Rehabil. 2012; 9:4.

28. Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997; 48:1398–403.

29. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006; 19:84–90.

30. Liepert J, Uhde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001; 248:315–21.

31. Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000; 31:1210–6.

33. Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007; 180:583–93.

Table 1.
Types and locations of lesions
Table 2.
Demographic and baseline clinical characteristics
| Real-rTMS group (n =8) | Sham-rTMS group (n = 8) | p-valuea) | |
|---|---|---|---|
| Age (yr) | 54.25±9.07 | 60.13±11.58 | 0.278 |
| Time since stroke onset (mo) | 3.19±2.14 | 3.94±1.37 | 0.418 |
| Fugl-Meyer Assessment score (out of 66) | 47.00±7.95 | 46.5±5.26 | 0.884 |
| Mini Mental State Examination score (out of 30) | 26.38±1.85 | 25.75±1.75 | 0.499 |
| Star Cancellation Test score | 53.13±3.83 | 53.25±2.60 | 0.940 |
| Imitation of meaningless gesture | 6.00±0.00 | 5.88±0.35 | 0.351 |
Table 3.
Corticospinal excitability of the non-lesioned hemisphere and performance of the paretic hand at baseline, post1, post2, post3, and post4
Table 4.
Correlations between changes in corticospinal excitability of the non-lesioned hemisphere and performance time of the can-lifting item of the paretic hand, compared at baseline, post1, post2, post3, and post4




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download