INTRODUCTION
MATERIALS AND METHODS
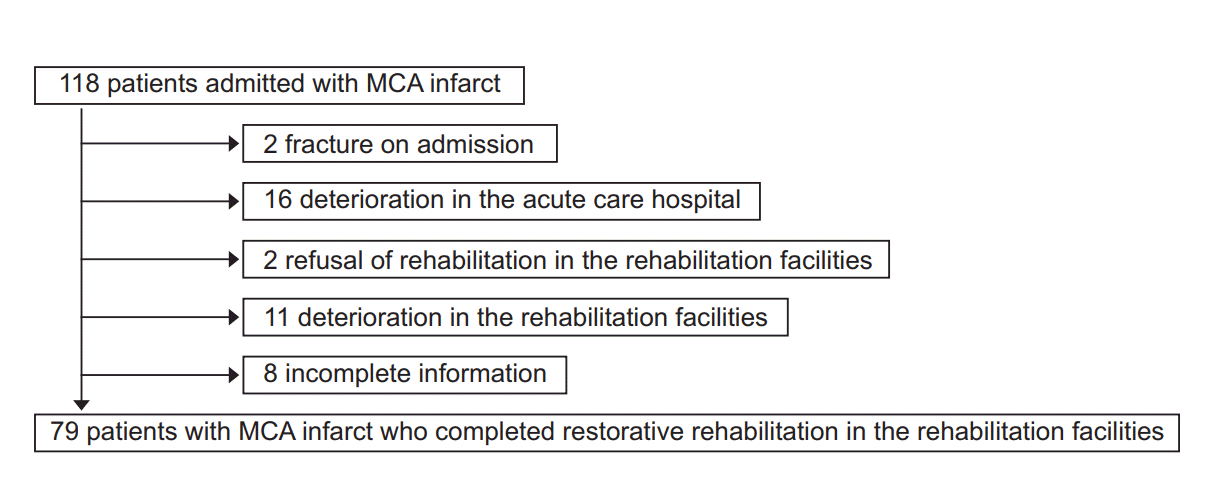
Patients
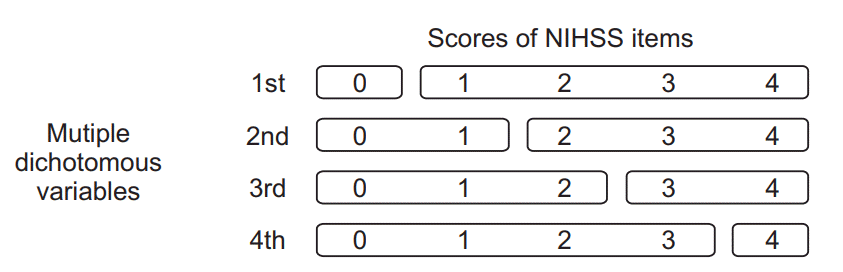
NIHSS and FIM measurements
Statistics
RESULTS
 | Fig. 3.Scatter diagrams showing the total score of the National Institutes of Health Stroke Scale on the x-axis, and independence or dependence of Functional Independence Measure defined in the text on the y-axis in 53 patients of the model-development group (1=independence, 0=dependence). |
Table 1.
| Model-development group (n=53) | Confirmatory group (n=26) | p-value | |
|---|---|---|---|
| Age (yr) | 74 (67–80) | 74 (61.25–78.5) | 0.75a) |
| Sex, male | 33 | 19 | 0.45b) |
| Modified Rankin Scale | 0 (0-1) | 0 (0–0.75) | 0.09a) |
| Laterality, right | 23 | 14 | 0.47b) |
| DWI–ASPECTS+W | 9 (9-10) | 9 (8.25–10) | 0.84a) |
| Infarction type | 0.99c) | ||
| Lacunar | 21 | 10 | |
| Atherome | 22 | 11 | |
| Cardiac emboli | 10 | 5 | |
| Recombinant tissue plasminogen activator therapy | 8 | 3 | 1.0b) |
| Removal of thrombosis of internal carotid artery | 1 | 0 | 1.0b) |
| Carotid artery stenting | 0 | 2 | 0.11b) |
| Botulinum toxin injection | 5 | 1 | 0.66b) |
| Length of stay in hospital (day) | |||
| Acute | 32 (26–38) | 30 (25–34.5) | 0.33a) |
| Rehabilitation | 105 (65–137) | 87.5 (45–141.5) | 0.50a) |
| Initial rehabilitation | 2 (1–2) | 2 (1–2) | 0.99a) |
| NIHSS total score | 9 (5–14) | 6 (4.25–11.25) | 0.06a) |
| FIM motor total score | 80 (51–87) | 78 (60–85.5) | 0.82a) |
| FIM cognition total score | 30 (19–35) | 27.5 (24–35) | 0.57a) |
| FIM total score | 110 (70–119) | 107.5 (84.5–118.75) | 0.80a) |
Table 2.
The plus score of multivariate logistic regression analyses means independence or supervision.
Predictive model was assessed using the logit of each multiple logistic regression equation and the real outcome.
FIM, Functional Independence Measure; mRS, modified Rankin Scale; LOC, level of consciousness.
Affected upper extremity, Affected lower extremity, mRS, Extinction and inattention, and Sensory 2nd: when these values are <2, they can be converted to ‘0’; ≥2, converted to ‘1’.
Affected upper extremity 3rd: when this value is <3, it can be converted to ‘0’; ≥3, converted to ‘1’.
mRS 4th: when this value is <4, it can be converted to ‘0’; ≥4, converted to ‘1’.
Table 3.
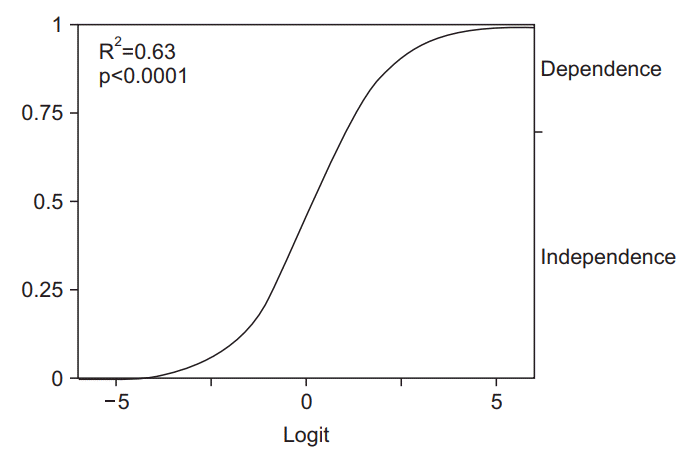 | Fig. 4.Example of logistic curve obtained from multivariate logistic model (bed transfer). The model equation is as follows: 19.63-(0.20×Age)-(3.39×Affected Upper Extremity 3rd)-(3.91×Affected Lower Extremity 2nd). Zero in the horizontal axis indicates a logistic probability 0.50. The curve showed the probability of bed transfer independence. The distance from the bottom to the top of the graph curve is the probability of independence. |
Table 4.
| FIM motor item |
Model-development group |
Confirmatory group |
||||||
|---|---|---|---|---|---|---|---|---|
| Independence/Supervision | Dependence/Physically assistance | Total | PCR×1.25 (%) | Independence/Supervision | Dependence/Physically assistance | Total | PCR×1.25 | |
| Eating (%) | 34/38 (89) | 9/15 (60) | 43/53 (81) | >78.7 | 17/19 (89) | 4/7 (57) | 21/26 (81) | >80.6 |
| Grooming | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.5 | 18/21 (86) | 5/5 (100) | 23/26 (88) | >71.7 |
| Bathinga) | 32/36 (89) | 16/17 (94) | 48/53 (91) | >66.3 | 16/21 (76) | 3/5 (60) | 19/26 (73) | >71.7 |
| Upper body dressing | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 18/24 (75) | 2/2 (100) | 20/26 (77) | >71.7 |
| Lower body dressing | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 18/24 (75) | 2/2 (100) | 20/26 (77) | >71.7 |
| Toileting | 34/37 (92) | 13/16 (81) | 47/53 (80) | >72.3 | 18/21 (86) | 4/5 (80) | 22/26 (85) | >75.8 |
| Bowel management | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 19/24 (79) | 2/2 (100) | 21/26 (81) | >75.8 |
| Bladder management | 37/39 (95) | 13/14 (93) | 50/53 (94) | >74.3 | 19/21 (90) | 4/5 (80) | 23/26 (88) | >80.6 |
| Bed transfer | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.3 | 17/21 (81) | 3/5 (60) | 20/26 (77) | >75.8 |
| Toilet transfer | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.3 | 17/21 (81) | 4/5 (80) | 21/26 (81) | >71.7 |
| Shower transfera) | 29/37 (78) | 14/16 (88) | 43/53 (81) | >64.3 | 15/21 (71) | 4/5 (80) | 19/26 (73) | >65.8 |
| Locomotion | 33/38 (87) | 13/15 (87) | 46/53 (87) | >72.3 | 17/21 (81) | 4/5 (80) | 21/26 (81) | >71.7 |
| Stair climba) | 28/38 (74) | 15/15 (100) | 43/53 (81) | >62.7 | 10/21 (48) | 5/5 (100) | 15/26 (58) | <65.8 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download