INTRODUCTION
MATERIALS AND METHODS
Study design and patients
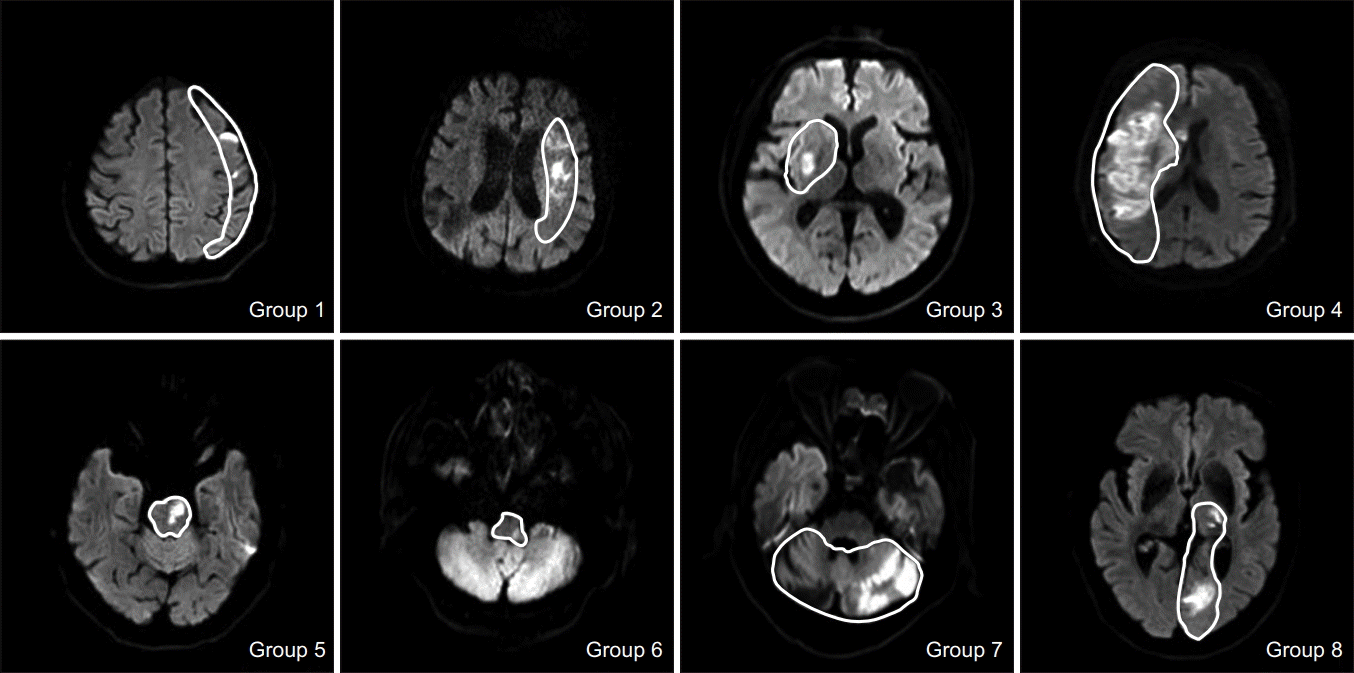 | Fig. 1.The lesions were divided into 8 groups that can be observed clinically. The line represents the area of each group. Group 1, middle cerebral artery (MCA) territory (cortical lesions); Group 2, MCA territory (subcortical lesions except for basal ganglia lesions); Group 3, basal ganglia lesions; Group 4, MCA territory lesions, including basal ganglia lesions; Group 5, pontine lesions; Group 6, medullary lesions; Group 7, cerebellar lesions; Group 8, posterior cerebral artery (PCA) territory lesions. |
Table 1.
| Variable | Overall | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | p-value | Post-hoc test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 275 | 37 | 17 | 75 | 52 | 45 | 17 | 12 | 20 | ||
| Sex (female:male) | 134:141 | 20:17 | 9:8 | 44:31 | 19:33 | 23:22 | 6:11 | 5:7 | 8:12 | 0.269a) | |
| Age (yr) | 72.1±9.9 | 76.7±7.6 | 74.7±5.9 | 71.1±10.6 | 70.1±9.8 | 74.8±8.6 | 64.9±12.2 | 67.6±12.2 | 73.6±7.7 | 0.000c) | 1>4,6,7 |
| Laterality (right:left) | 139:136 | 12:25 | 11:6 | 40:35 | 26:26 | 27:18 | 7:10 | 8:4 | 8:12 | 0.147a) | |
| Hypertension | 155 (56.4) | 22 (59.5) | 9 (52.9) | 43 (57.3) | 22 (42.3) | 33 (73.3) | 7 (41.2) | 8 (66.7) | 11 (55.0) | 0.106a) | |
| DM | 75 (27.3) | 10 (27.0) | 3 (17.6) | 22 (29.3) | 12 (23.1) | 16 (35.6) | 3 (17.6) | 4 (33.3) | 5 (25.0) | 0.804b) | |
| K-MMSE | 19.4±7.0 | 13.3±8.3 | 16.3±6.5 | 21.7±5.6 | 17.0±7.5 | 21.8±5.5 | 24.2±6.7 | 18.9±6.0 | 16.4±6.4 | 0.000c) | 1<3,5,6 |
| 4,8<6 | |||||||||||
| 3>4 | |||||||||||
| K-MBI | 29.5±23.0 | 24.0±19.7 | 27.0±24.7 | 33.7±20.9 | 21.7±22.0 | 35.4±25.0 | 37.2±27.0 | 32.3±29.3 | 24.4±18.1 | 0.009c) | 3>4 |
| Diet, tube feeding | 17 (45.9) | 7 (41.2) | 19 (25.3) | 23 (44.2) | 16 (35.6) | 9 (52.9) | 7 (58.3) | 2 (10.0) | 0.010a) | ||
| Diet, dysphagia diet | 15 (40.5) | 4 (23.5) | 24 (32.0) | 13 (25.0) | 11 (24.4) | 6 (35.3) | 1 (8.3) | 11 (55.0) | |||
| Diet, regular diet | 5 (13.5) | 6 (35.3) | 32 (42.7) | 16 (30.8) | 18 (40.0) | 2 (11.8) | 4 (33.3) | 7 (35.0) |
Values are presented as mean±standard deviation or frequency (%).
1, MCA territory (cortical); 2, MCA territory (subcortical except basal ganglia); 3, basal ganglia; 4, basal ganglia and MCA territory; 5, Pons; 6, Medullary; 7, cerebellum; 8, PCA territory; MCA, middle cerebral artery; PCA, posterior cerebral artery; DM, diabetes mellitus; K-MMSE: Korean version of Mini-Mental Status Examination; K-MBI, Korean version of Modified Barthel Index.
Swallowing assessment
Outcome assessment
Statistical methods
RESULTS
Table 2.
| Variable | 1 (n=37) | 2 (n=17) | 3 (n=75) | 4 (n=52) | 5 (n=45) | 6 (n=17) | 7 (n=12) | 8 (n=20) | p-valuea) | Post-hoc test |
|---|---|---|---|---|---|---|---|---|---|---|
| VFSS score | 36.5 (11–76) | 30.5 (14-66.5) | 38.5 (9.5-80) | 37 (4.5–72) | 38.5 (9.5–83) | 50.5 (17–89.5) | 45.5 (15–57.5) | 29.75 (10–60.5) | 0.076 | N/A |
| Lip close | 0 (0–4) | 0 (0–4) | 0 (0–4) | 0 (0–4) | 0 (0–4) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.741 | N/A |
| Bolus formation | 3 (0–6) | 3 (0–3) | 3 (0–6) | 3 (0–6) | 3 (0–6) | 3 (0–6) | 3 (0–3) | 3 (0–6) | 0.139 | N/A |
| Mastication | 4 (0–8) | 4 (0–8) | 4 (0–8) | 4 (0–10) | 0 (0–8) | 0 (0–8) | 4 (0–4) | 4 (0–8) | 0.049 | 2>5 |
| Premature bolus loss | 1.5 (0–4.5) | 1.5 (0–4.5) | 1.5 (0–4.5) | 1.5 (0–4.5) | 1.5 (0–4.5) | 3 (0–4.5) | 2.25 (0–3) | 1.5 (0–3) | 0.327 | N/A |
| Apraxia | 3 (0–4.5) | 3 (0–4.5) | 1.5 (0–4.5) | 2.25 (0–4.5) | 1.5 (0–4.5) | 1.5 (0–4.5) | 3 (0–4.5) | 1.5 (0–3) | 0.001 | 1>3 |
| Aspiration | 12 (0–12) | 12 (0–12) | 6 (0–12) | 12 (0–12) | 12 (0–12) | 12 (0–12) | 9 (6–12) | 12 (0–12) | 0.108 | N/A |
| Laryngeal elevation | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0 (0–9) | 0.988 | N/A |
| Oral transit time | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 0.213 | N/A |
| Vallecular residue | 2 (0–6) | 2 (0–4) | 2 (0–6) | 2 (0–4) | 2 (0–6) | 2 (2–6) | 2 (0–6) | 2 (0–4) | 0.000 | 1,2,4,8<5,6 |
| 3<6 | ||||||||||
| Pyriform sinuses residue | 0 (0–9) | 0 (0–9) | 4.5 (0–13.5) | 0 (0–13.5) | 4.5 (0–9) | 9 (0–13.5) | 4.5 (–9) | 0 (0–9) | 0.000 | 1,2,4<6 |
| 1,4<3 | ||||||||||
| Coating of pharyngeal wall | 9 (0–9) | 9 (0–9) | 9 (0–9) | 9 (0–9) | 9 (0–9) | 9 (0–9) | 9 (0–9) | 0 (0–9) | 0.003 | 3,5,6<8 |
| Tongue to palate contact | 0 (0–10) | 0 (0–5) | 0 (0–-5) | 0 (0–10) | 0 (0–5) | 0 (0–5) | 0 (0–5) | 0 (0–5) | 0.000 | 2,4<3 |
| Triggering of pharyngeal swallow | 0 (0–3) | 0 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 3 (0–3) | 1.5 (0–3) | 0.387 | N/A |
| Pharyngeal transit time | 0 (0–6) | 0 (0–6) | 0 (0–6) | 0 (0–6) | 0 (0–6) | 6 (0–6) | 3 (0–6) | 0 (0–6) | 0.310 | N/A |
Values are presented as median (minimum–maximum).
1, MCA territory (cortical); 2, MCA territory (subcortical except basal ganglia); 3, basal ganglia; 4, basal ganglia and MCA territory; 5, Pons; 6, Medullary; 7, cerebellum; 8, PCA territory; MCA, middle cerebral artery; PCA, posterior cerebral artery; VFSS, videofluoroscopic swallowing study; N/A, not applicable.
Table 3.
| Variable | B | 95% CI | β | p-valuea) | VIF |
|---|---|---|---|---|---|
| Sex (male) | 7.766 | 2.446 to 13.086 | 0.217 | 0.004 | 1.107 |
| Age | 0.127 | -0.146 to 0.400 | 0.071 | 0.359 | 1.163 |
| Laterality (right) | -2.281 | -7.613 to 3.051 | -0.063 | 0.400 | 1.098 |
| Hypertension (yes) | 1.267 | -3.943 to 6.478 | 0.035 | 0.632 | 1.038 |
| DM (yes) | -2.590 | -8.482 to 3.301 | -0.063 | 0.387 | 1.026 |
| K-MMSE | -0.105 | -0.529 to 0.319 | -0.041 | 0.627 | 1.381 |
| K-MBI | -0.138 | -0.258 to -0.017 | -0.182 | 0.025 | 1.273 |
VDS, videofluoroscopic dysphagia scale; VFSS, videofluoroscopic swallowing study; B, unstandardized regression efficient; CI, confidence interval; β, standardized regression coefficient; VIF, variance inflation index; DM, diabetes mellitus; K-MMSE, Korean version of Mini-Mental State Examination; K-MBI, Korean version of Modified Barthel Index.
Table 4.
| Parameter | OR | 95% CI | p-valuea) |
|---|---|---|---|
| Lip close | |||
| K-MBI | 0.959 | 0.933–0.986 | 0.003 |
| Bolus formation | |||
| Laterality (right) | 0.421 | 0.197–0.898 | 0.025 |
| Hypertension (yes) | 2.172 | 1.067–4.419 | 0.032 |
| Mastication | |||
| K-MMSE | 0.950 | 0.905–0.998 | 0.040 |
| K-MBI | 0.985 | 0.971–0.999 | 0.036 |
| Premature bolus loss | |||
| K-MBI | 0.987 | 0.975–1.000 | 0.051 |
| Apraxia | |||
| K-MBI | 0.980 | 0.966–0.994 | 0.006 |
| Reduced laryngeal elevation and epiglottic closure | |||
| K-MBI | 0.981 | 0.966–0.997 | 0.020 |
| Oral transit time | |||
| K-MBI | 0.987 | 0.974–1.000 | 0.047 |
| Residue on the valleculae | |||
| Laterality (right) | 0.476 | 0.227–1.000 | 0.050 |
| Residue in the pyriform sinuses | |||
| Sex (male) | 4.684 | 2.405–9.123 | 0.000 |
| Age | 1.037 | 1.003–1.072 | 0.034 |
| Coating of pharyngeal wall after swallow | |||
| Laterality (right) | 0.520 | 0.269–1.006 | 0.052 |
| Pharyngeal delay time | |||
| K-MBI | 0.982 | 0.970–0.995 | 0.007 |
| Pharyngeal transit time | |||
| Sex (male) | 2.029 | 1.089–3.782 | 0.026 |
| K-MBI | 0.986 | 0.972–0.999 | 0.038 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download