Abstract
Objective
To determine if there is muscle mass reduction in patients with ankylosing spondylitis (AS) compared to the general population and to examine the relationship between skeletal muscle mass, quality of life (QOL), strength, and mobility in patients with AS.
Methods
A total of 30 AS patients were enrolled in this study. Skeletal muscle mass was measured by bioelectrical impedance analysis, and it was expressed as the skeletal muscle mass index (SMI). QOL was assessed using the EuroQOL (EQ-5D). To measure mobility, the modified Schöber test and chest expansion test were used. To measure grip strength as a measure of muscle strength, we used the hydraulic hand dynamometer. Additionally, we divided the patients into two groups according to the degree of X-ray finding and compared the differences between the two groups.
Results
There was no significant reduction in skeletal muscle mass in patients with AS compared to the general population. Also, there was no significant correlation between SMI and QOL. On the other hand, there was a significant positive correlation between SMI and mobility, and grip strength. A significant positive correlation was found between mobility and QOL. Additionally, there was a statistically significant difference in mobility between the two groups according to the degree of X-ray finding.
Ankylosing spondylitis (AS) is a systemic inflammatory rheumatic disease, which can result in pain along with loss of physical ability and quality of life (QOL), and it is characterized by prominent inflammation and progressive stiffness of the spine. Primarily, the spine is affected by AS and peripheral arthritis is usually monoarticular or oligoarticular.
Functional impairment increases with age, disease duration, and severity of symptoms. There was a significant relationship between impairment and limitation of activity in AS patients [1]. Frequent pulmonary involvement in patients with AS affecting the QOL was also shown previously [23]. Bony ankylosis of the thoracic vertebrae and sternal joints results in limitation of chest wall expansion [4]. Spinal mobility may have a predictive value for pulmonary impairment in patients with AS [5].
Recent studies have reported both sarcopenia and decrease in muscle strength among patients with chronic inflammatory diseases such as rheumatoid arthritis [6]. There are concerns that sarcopenia might affect exercise tolerance, physical fitness, and eventually have an adverse effect on cardiovascular fitness, and physical and emotional well-being [789]. Several studies have shown that muscle fatigue can cause muscle weakness and it can also affect the postural control [10]. Marcora et al. [11] showed that patients with well-established AS had a significant reduction of 12% in lean mass of the arms and legs. However, previous studies on body composition in patients with AS have been published and they did not consistently show a reduction in muscle mass [121314]. Thus, studies on muscle mass in AS patients are still debated.
A previous study reported that maintaining physical activity and adequate pain management in AS patients is important for improving QOL [5]. However, to the best of our knowledge, none of the studies have reported the association between skeletal muscle mass and QOL in AS patients. Therefore, this study was conducted to determine if there was a decrease in muscle mass in patients with AS compared to the general population and to examine the relationship between skeletal muscle mass and QOL in patients with AS. The relationship between skeletal muscle mass, strength, and mobility was also evaluated. In addition, the patients were divided into two groups according to the degree of X-ray finding and disease duration, and the variables were compared between the two groups.
A total of 38 patients who were definitely diagnosed with AS and were admitted to the outpatient clinic of the Department of Physical Medicine and Rehabilitation were consecutively assessed in this study. Of these 38 patients, 30 were males and 8 were females. Because the normal values of male and female skeletal muscle mass indexes (SMIs) were different, 30 males were selected as the final subjects. Patients were divided into the sacroiliitis group and the squaring group depending on the degree of X-ray finding. All patients voluntarily agreed to give their informed consent and the research was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB no. 2014-04-018). Patients were selected according to the modified New York criteria [15] and characteristics were recorded. Patients with cardiac or pulmonary disease, those who had serious knee or foot injury, and those who had some other systemic disease were excluded from this study group.
To measure skeletal muscle mass, we used the InBody S10 (Biospace, Seoul, Korea), which is a device that performs bioelectrical impedance measurement (Fig. 1). After height and weight were measured, four electrodes were attached to both upper and lower extremities in the supine position. As the index of muscle mass, we used the SMI, which was calculated as follows:
Skeletal muscle mass is the sum of appendicular muscle mass and trunk muscle mass.
Additionally, to evaluate QOL, the EuroQOL (EQ-5D) was used. The EuroQOL (EQ-5D) generic health index comprises a five-part questionnaire and a visual analogue self-rating scale. The questionnaire may be used as a health index to calculate the ‘utility’ value or as a health profile [16].
The modified Schöber test and chest expansion test were used to measure mobility. The modified Schöber test (Fig. 2) is a method frequently used to assess the active range of motion of the lumbar spine. The lumbosacral junction acts as the landmark, the second line is marked 10 cm above the first line, and the third line is marked 5 cm below the first line. The difference between the measurements in erect and flexion positions indicates the outcome of lumbar flexion. If the distance between the two points does not increase by more than 20 cm, it is a sign of restriction in lumbar mobility.
Limitation of chest expansion to 2.5 cm or less is one of the significant clinical factors in the modified New York criteria [17]. Chest expansion test (Fig. 3) was performed by obtaining measurements with a tape measure placed circumferentially around the chest wall at the fourth intercostal space [18].
The hydraulic hand dynamometer (Seahan Corp., Masan, Korea) was used to measure the grip strength as a measure of muscle strength. Grip strength was measured using the standard procedure proposed by the Korean Association of Occupational Therapists. The grip strength measurement method was as follows: for each of the tests for hand strength, the subjects were seated with the shoulder adducted and neutrally rotated, elbow flexed at 90°, forearm in a neutral position, and wrist between 0° and 30° dorsiflexion and between 0° and 15° ulnar deviation. For each strength test, the scores of three successive trials were recorded for each hand. The average value measured three times in total was recorded [19].
To draw the correlation between muscle mass, QOL, strength, and mobility, the Spearman correlation coefficient was used. The Student t-test was used to compare the two groups categorized by X-ray finding and disease duration. When the p-value was less than 0.05, it was considered to indicate statistical significance. The SPSS program ver. 20.0 for Windows (IBM, Armonk, NY, USA) was used for data analysis.
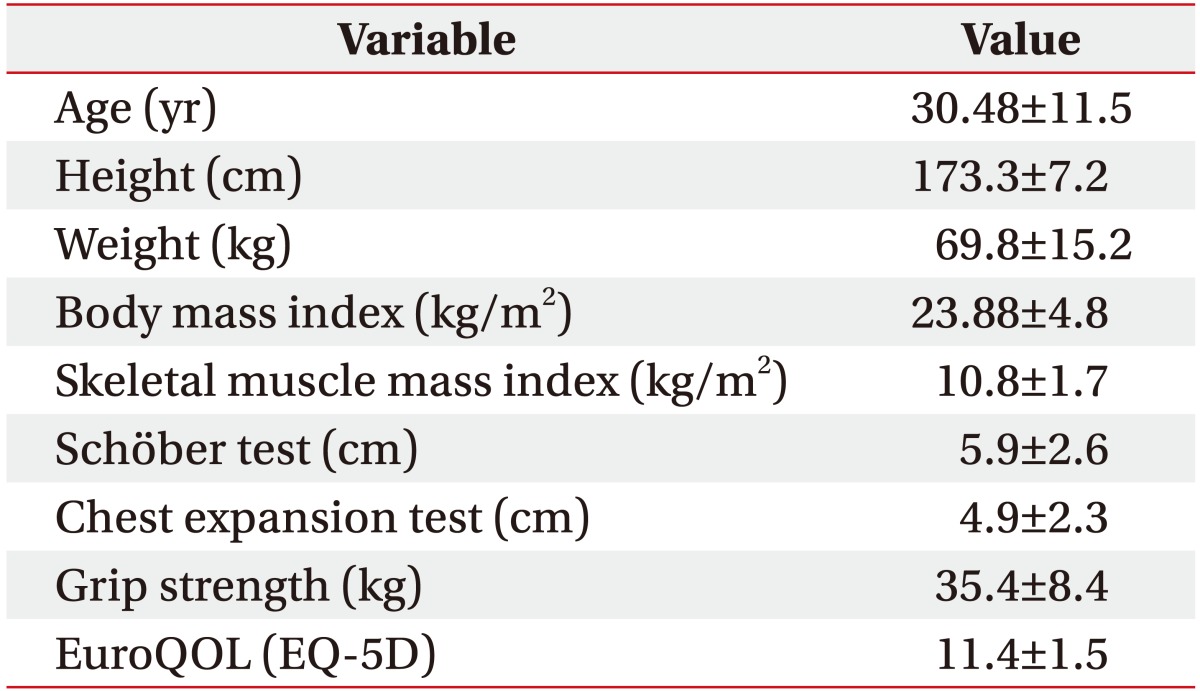
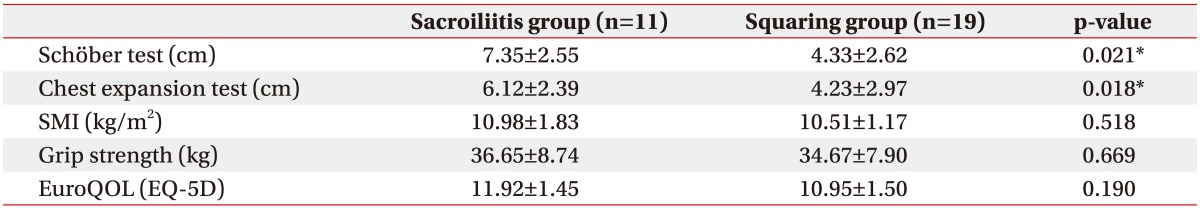
The demographic data including test results are shown in Table 1. Subjects were 30 men with a mean age of 30.5 years (range, 17–58 years) at the time of this study. The mean length obtained with Schöber test and chest expansion test was 5.9 cm and 4.9 cm, respectively. The remaining data are shown in Table 1.
The SMIs of subjects only in their 20s and 30s were compared with those of the general population because the number of patients included in the study was small. There was no significant reduction in muscle mass in patients with AS compared to the general population. The SMIs of subjects in their 20s and 30s who were included in this study was 10.7±1.3 and 11.4±2.4 kg/m2, and the SMIs of the general population was 10.9±1.2 and 11.0±1.1 kg/m2, respectively. The SMIs of the general population were provided by the InBody Corporation. The data on SMIs provided by InBody Corporation was derived from 10,000 Korean adults.
In the Spearman correlation, there was no statistically significant correlation between SMI and QOL (correlation coefficient=0.290, p=0.120). There was a significant positive correlation between SMI and Schöber test/chest expansion test (correlation coefficient=0.475/0.401, p=0.008/0.028). Grip strength was significantly correlated with SMI (correlation coefficient=0.376, p=0.041) as expected, but it was not significantly correlated with Schöber test and chest expansion test. Additionally, a significant positive correlation was found between QOL and Schöber test/chest expansion test (correlation coefficient= 0.441/0.410, p=0.015/0.024) (Table 2).
In the Student t-test, there was a statistically significant difference in Schöber test and chest expansion test between the sacroiliitis group and the squaring group categorized by X-ray finding (Table 3).
AS is a chronic systemic inflammatory rheumatic disease which primarily affects the axial skeletal system via an unknown pathological mechanism. Depending on the course of the disease, AS progressively limits spinal and thoracic mobility and causes serious functional impairment [4]. As a consequence, patients with AS experience limitations in physical activities and reduced QOL [20]. Given the chronic inflammation and risk of reduced physical activity, patients are also at risk for accelerated muscle loss [14]. The consensus for muscle mass reduction in the body composition of AS patients, one of the chronic inflammatory diseases, has not yet been established [11121314]. Sarcopenia seems to be associated with many harmful effects on life. Sarcopenia represents an impaired state of health with a high personal toll—mobility disorders, increased risk of falls and fractures, impaired ability to perform activities of daily living, disabilities, loss of independence, and increased risk of death [2122232425].
A consensus for the reduction of muscle mass in AS patients has not yet been established. In this study, there was no significant reduction in muscle mass in patients with AS compared to the general population. The reason for this result is presumed to be related to disease severity and duration. Of the patients included in this study, only 2 patients had syndesmophytes, accounting for 6.7% of the total patient population. Previous studies have reported that there is no difference in muscle mass between AS patients and the general population, as observed in this study. One of these studies excluded patients with syndesmophytes [26], while in another study, 27% of the patients had syndesmophytes [12]. On the other hand, in contrast to the results of this study, the other study showed that muscle mass in AS patients is reduced compared to that in the general population [11]. There were syndesmophytes in 84% of the patients included. These findings suggest that a high percentage of patients with syndesmophytes may result in changes in body composition. It was thought that there was no significant difference in skeletal muscle mass compared with the general population because the percentage of patients with syndesmophytes was as low as 6.7% in this study. In terms of disease duration, some interesting points were noted. The mean duration of disease was 11 years and 8 years, respectively, in the two studies in which there was no difference in muscle mass between the AS patients and the general population [1226]. On the other hand, the study, which showed that there was a difference in muscle mass, had a mean disease duration of 19 years [11]. In our study, mean disease duration was approximately 6 years; therefore, we may not have noted any difference in muscle mass in AS patients compared to the general population. This result may have also been obtained as the subjects included in this study were those patients who were referred to the outpatient clinic and who were relatively well managed.
There was no significant association between SMI and QOL. A previous study reported a positive correlation between fat-free mass index and physical activity in patients with AS [14]. Another study showed a significant correlation between lean mass and QOL in fibromyalgia patients [27]. A recent study reported that managing pain and maintaining physical activity is important for improving QOL in AS patients [5]. However, to the best of our knowledge, none of the studies have evaluated the association between skeletal muscle mass and QOL in AS patients. We think that our study is meaningful because it is the first study to evaluate the association between SMI and QOL, which is a key strength of our study. This study shows that there is no statistical significance between skeletal muscle mass and QOL in AS patients. The reason for these findings is that the patients included in this study did not have a significant reduction in muscle mass. The majority of the patients were not in the early stage of the disease because of squaring on X-ray, but the number of patients with syndesmophytes was low and the mean disease duration of 6 years was not very long. In addition, since the patients were recruited from outpatient clinics, it is likely that many of them were well managed. Although this study did not show a relationship between skeletal muscle mass and QOL, more studies with a larger sample size are needed to confirm this finding and its relevance for the disease management.
We could identify a significant association between SMI and Schöber test, and chest expansion test. A previous study by Ince et al. [28] showed that stretching exercise is important to improve mobility in AS patients. Thus, previous studies have emphasized stretching exercise in AS patients' exercise programs because AS patients have reduced mobility, which decreases their QOL. This study showed that the reduction of mobility is associated with a reduction in muscle mass, and therefore, strengthening exercise might be important to prevent muscle mass reduction. Furthermore, AS patients' exercise programs should include strengthening exercises.
This study is meaningful as the patients were divided into two groups (sacroiliitis group vs. squaring group) according to the degree of X-ray finding, and the differences between the two groups were evaluated. There was a statistically significant difference in the Schöber test and chest expansion test between the group with sacroiliitis and squared spine and the group with sacroiliitis only. This indicates that changes in the spine itself on the X-ray may affect mobility. The SMI value was higher in the sacroiliitis group than in the squaring group (10.98±1.83 vs. 10.51±1.17 kg/m2), but there was no statistically significant difference. We hope that further studies including more patients will further evaluate the association between X-ray finding and SMI.
As mentioned before, this study is significant because it is the first study to evaluate the association between skeletal muscle mass and QOL in patients with AS, which is a key strength of our study. Also, this study is meaningful as the patients were divided into two groups according to X-ray finding, and then, the two groups were compared. To the best of our knowledge, none of the previous studies have compared the two groups according to X-ray finding in AS patients. In addition, in a situation where there is a debate on the reduction of muscle mass in patients with AS compared to the general population, our study is meaningful as it provides a possible answer. Finally, although further study on strengthening exercise is warranted, this study is meaningful as it is the first study to show that strengthening exercise may be helpful in the management of AS patients.
The most important limitation of this study is the low number of patients included in the study. Further studies involving various categories of patients in terms of disease severity and duration are needed. Another limitation of this study is that in this study, muscle mass was calculated as the sum of appendicular muscle mass and trunk muscle mass. AS is a disease that mainly involves the axial joints. However, the muscle mass value in this study is limited with respect to reflecting the axial muscle. Therefore, further studies on the relationship between axial muscle mass and other variables are needed.
In conclusion, maintaining muscle mass in AS patients may not be helpful for improving QOL, but it may contribute to achieving adequate mobility and strength. These findings suggests that a strengthening exercise may be needed to maintain and increase the muscle mass in AS patients. Further studies on strengthening exercise in AS patients are needed to confirm these findings.
ACKNOWLEDGMENTS
This work was supported by Institute for Information & communications Technology Promotion (IITP) grant funded by the Korea government (MSIP) (No.2017-0-01800,Development of AR sports training platform on smart glass based motion recognition).
References
1. Ozdem Yr O, Inanici F, Hascelik Z. Reduced vital capacity leads to exercise intolerance in patients with ankylosing spondylitis. Eur J Phys Rehabil Med. 2011; 47:391–397. PMID: 21364507.
2. Feltelius N, Hedenstrom H, Hillerdal G, Hallgren R. Pulmonary involvement in ankylosing spondylitis. Ann Rheum Dis. 1986; 45:736–740. PMID: 3767460.

3. Sampaio-Barros PD, Cerqueira EM, Rezende SM, Maeda L, Conde RA, Zanardi VA, et al. Pulmonary involvement in ankylosing spondylitis. Clin Rheumatol. 2007; 26:225–230. PMID: 16572281.

4. Durmuş D, Alayli G, Uzun O, Tander B, Canturk F, Bek Y, et al. Effects of two exercise interventions on pulmonary functions in the patients with ankylosing spondylitis. Joint Bone Spine. 2009; 76:150–155. PMID: 19084457.

5. Cho H, Kim T, Kim TH, Lee S, Lee KH. Spinal mobility, vertebral squaring, pulmonary function, pain, fatigue, and quality of life in patients with ankylosing spondylitis. Ann Rehabil Med. 2013; 37:675–682. PMID: 24236255.

6. Beenakker KG, Ling CH, Meskers CG, de Craen AJ, Stijnen T, Westendorp RG, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev. 2010; 9:431–436. PMID: 20553969.

7. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001; 137:231–243. PMID: 11283518.

8. Roubenoff R. Origins and clinical relevance of sarcopenia. Can J Appl Physiol. 2001; 26:78–89. PMID: 11291626.

9. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998; 147:755–763. PMID: 9554417.

10. Sahin N, Ozcan E, Baskent A, Karan A, Ekmeci O, Kasikcioglu E. Isokinetic evaluation of ankle muscle strength and fatigue in patients with ankylosing spondylitis. Eur J Phys Rehabil Med. 2011; 47:399–405. PMID: 21364512.
11. Marcora S, Casanova F, Williams E, Jones J, Elamanchi R, Lemmey A. Preliminary evidence for cachexia in patients with well-established ankylosing spondylitis. Rheumatology (Oxford). 2006; 45:1385–1388. PMID: 16603581.

12. Toussirot E, Michel F, Wendling D. Bone density, ultrasound measurements and body composition in early ankylosing spondylitis. Rheumatology (Oxford). 2001; 40:882–888. PMID: 11511757.

13. Sari I, Demir T, Kozaci LD, Akar S, Kavak T, Birlik M, et al. Body composition, insulin, and leptin levels in patients with ankylosing spondylitis. Clin Rheumatol. 2007; 26:1427–1432. PMID: 17260105.

14. Plasqui G, Boonen A, Geusens P, Kroot EJ, Starmans M, van der Linden S. Physical activity and body composition in patients with ankylosing spondylitis. Arthritis Care Res (Hoboken). 2012; 64:101–107. PMID: 22213726.

15. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–368. PMID: 6231933.
16. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol. 1997; 36:551–559. PMID: 9189057.

17. Bennett PH, Burch TA. New York symposium on population studies in the rheumatic diseases: new diagnostic criteria. Bull Rheum Dis. 1967; 17:453–458.
18. Moll JM, Wright V. An objective clinical study of chest expansion. Ann Rheum Dis. 1972; 31:1–8. PMID: 5008463.

19. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985; 66:69–74. PMID: 3970660.
20. Davis JC, van der Heijde D, Dougados M, Woolley JM. Reductions in health-related quality of life in patients with ankylosing spondylitis and improvements with etanercept therapy. Arthritis Rheum. 2005; 53:494–501. PMID: 16082640.

21. Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008; 12:433–450. PMID: 18615225.

22. Hartman MJ, Fields DA, Byrne NM, Hunter GR. Resistance training improves metabolic economy during functional tasks in older adults. J Strength Cond Res. 2007; 21:91–95. PMID: 17313273.

23. Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007; 55:1216–1223. PMID: 17661960.

24. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003; 95:1851–1860. PMID: 14555665.

25. Topinkova E. Aging, disability and frailty. Ann Nutr Metab. 2008; 52(Suppl 1):6–11. PMID: 18382070.

26. Dos Santos FP, Constantin A, Laroche M, Destombes F, Bernard J, Mazieres B, et al. Whole body and regional bone mineral density in ankylosing spondylitis. J Rheumatol. 2001; 28:547–549. PMID: 11296956.
27. Arranz L, Canela MA, Rafecas M. Relationship between body mass index, fat mass and lean mass with SF-36 quality of life scores in a group of fibromyalgia patients. Rheumatol Int. 2012; 32:3605–3611. PMID: 22095395.

28. Ince G, Sarpel T, Durgun B, Erdogan S. Effects of a multimodal exercise program for people with ankylosing spondylitis. Phys Ther. 2006; 86:924–935. PMID: 16813473.

Fig. 1
Skeletal muscle mass was measured using bioelectrical impedance analysis (InBody S10; Biospace, Seoul, Korea). Surface electrodes were attached on both ankles and fingers (thumb and middle fingers).
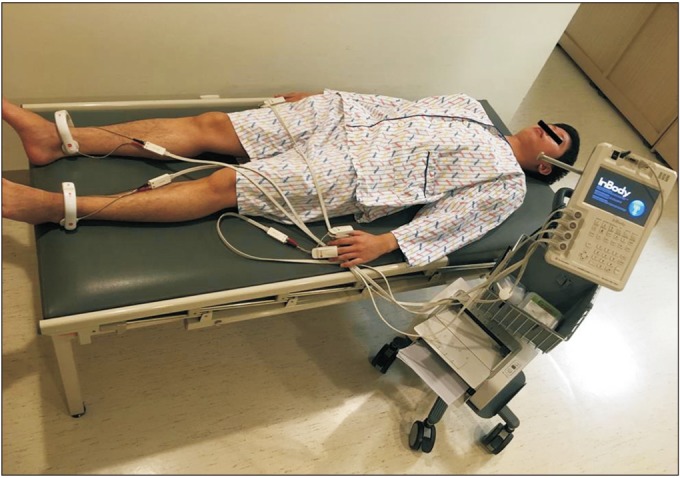
Fig. 2
Modified Schöber test. With the patient standing upright, a mark was placed at the lumbosacral junction, and further marks were placed 5 cm below and 10 cm above (A). The patient was then asked to bend forward as far as possible, and the distance between the two marks was measured again (B).
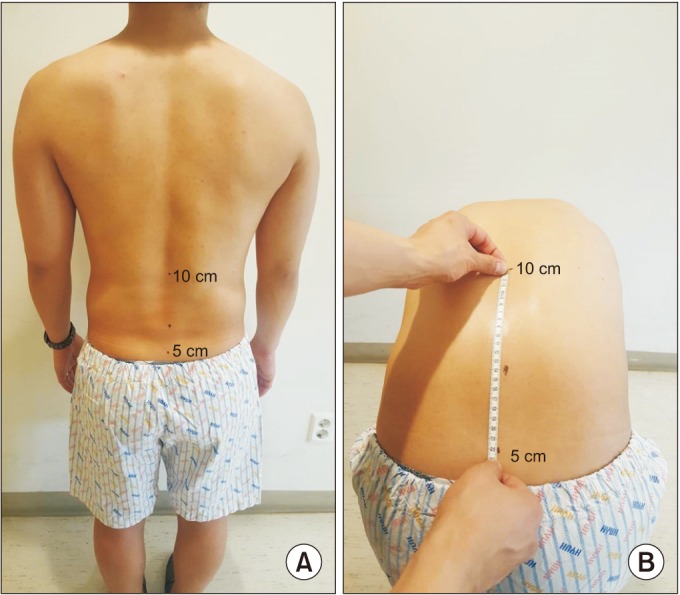
Fig. 3
Chest expansion test. Chest expansion was measured as the difference between maximal inspiration and maximal forced expiration at the fourth intercostal space in males or just below the breasts in females.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



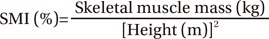

 XML Download
XML Download