Abstract
Objective
To determine whether providing education about the disease pathophysiology and drug mechanisms and side effects, would be effective for reducing the use of pain medication while appropriately managing neurogenic pain in spinal cord injury (SCI) patients.
Methods
In this prospective study, 109 patients with an SCI and neuropathic pain, participated in an educational pain management program. This comprehensive program was specifically created, for patients with an SCI and neuropathic pain. It consisted of 6 sessions, including educational training, over a 6-week period.
Results
Of 109 patients, 79 (72.5%) initially took more than two types of pain medication, and this decreased to 36 (33.0%) after the educational pain management program was completed. The mean pain scale score and the number of pain medications decreased, compared to the baseline values. Compared to the non-response group, the response group had a shorter duration of pain onset (p=0.004), and a higher initial number of different medications (p<0.001) and certain types of medications.
Neuropathic pain is a common and significant problem, for individuals with a spinal cord injury (SCI). The reported prevalence of neuropathic pain after an SCI varies from 38% to 40% [12]. Neuropathic pain is likely to persist, and it tends to worsen [3]. It has been correlated with a depressive mood [3456], poorer rehabilitation outcome [7], and lower quality of life [89]. Although various medical treatments are used, the treatment of SCI-related neuropathic pain remains a clinical challenge.
Various pharmacological agents such as gabapentin, pregabalin, and amitriptyline have been suggested for treating neuropathic pain. However, there is controversy regarding the effects of medications on SCI-related neuropathic pain, due to a lack of clear evidence [1011]. A pharmacologic intervention is often inadequate, resulting in only a reduction of 20%–30% in the pain intensity [12]. Furthermore, neuropathic pain is associated with polypharmacy [10131415], and the increased use of medications can lead to increased costs, more side effects, and an increased risk of fatigue as well as confused states, falls, and injuries [11]. High rates of polypharmacy and positive associations with drug-related problems in the population with an SCI have been reported, and medications for pain increase the risk of fatigue [16].
Therefore, there is growing interest in non-pharmacological treatments, as these can be used as an effective complement to pharmacological interventions to enhance the overall impact of pain-reliving interventions for individuals with an SCI [17]. In addition, since patient education does not require special equipment, providing continuous feedback alone may be effective for reducing the use of polypharmacy [18]. It has been suggested that improving and maintaining sleep states or environments are more effective than drug therapy or using treatment modalities, but scant research data is available to substantiate this.
In our study, we explored whether providing education about the disease pathophysiology and drug mechanisms and side effects would be effective, for reducing the use of pain medication while appropriately managing pain in patients with an SCI and neuropathic pain.
Between January 2014 and December 2014, all patients with an SCI admitted to the Department of Physical Medicine and Rehabilitation at Yonsei University Hospital were screened. Clinical data were examined, and the International Spinal Cord Injury Pain Classification (ISCIPC) was used. They were included in the study when they met the following inclusion criteria: (1) patients older than 20, (2) those with symptom onset of less than 6 months, (3) patients with a non-progressive spinal cord lesion due to traumatic or non-traumatic causes, (4) those with neuropathic pain, as defined by the ISCIPC, and (5) patients with taking pain medication. Exclusion criteria were patients (1) with musculoskeletal pain; (2) concurrent, multiple bony fractures, except spine fractures; (3) a previous brain injury or concurrent brain lesion, (4) history of neuropathic pain, and (5) previous neuropsychiatric disorder.
Subjects were assessed within 48 hours of the beginning, and end of the intervention period. Subjects' clinical data, including demographic characteristics such as sex and age, and time since injury, were collected. The assessment of neurological function after SCI was performed according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), and subjects' Neurological Level of Injury (NLI), ASIA impairment scale (AIS) grade, and injury etiology were collected.
According to the ISCIPC, a detailed clinical pain history that included onset, pain description (numbness, pricking sense, etc.), course and daily variation, associated symptoms, and factors was investigated; additionally, physical examination was also conducted that involved confirmation by a painful response and character of the pain, such as allodynia, hyperalgesia, hyperalgesia, etc. Neuropathic pain was divided into three groups: at-level neuropathic pain, below-level neuropathic pain, other (e.g., complex regional pain syndrome) [1920]. A rehabilitation doctor matched subjects' pain drawings, which illustrated the worst pain site and pain severity with pain characteristics according to the classification. Patients were asked to rate their global intensity of pain using the visual analog scale (VAS) (0, no pain; 10, worst possible pain) weekly, and they maintained a pain diary; scores were averaged over seven days. The main outcome was pain at baseline and 6 weeks later.
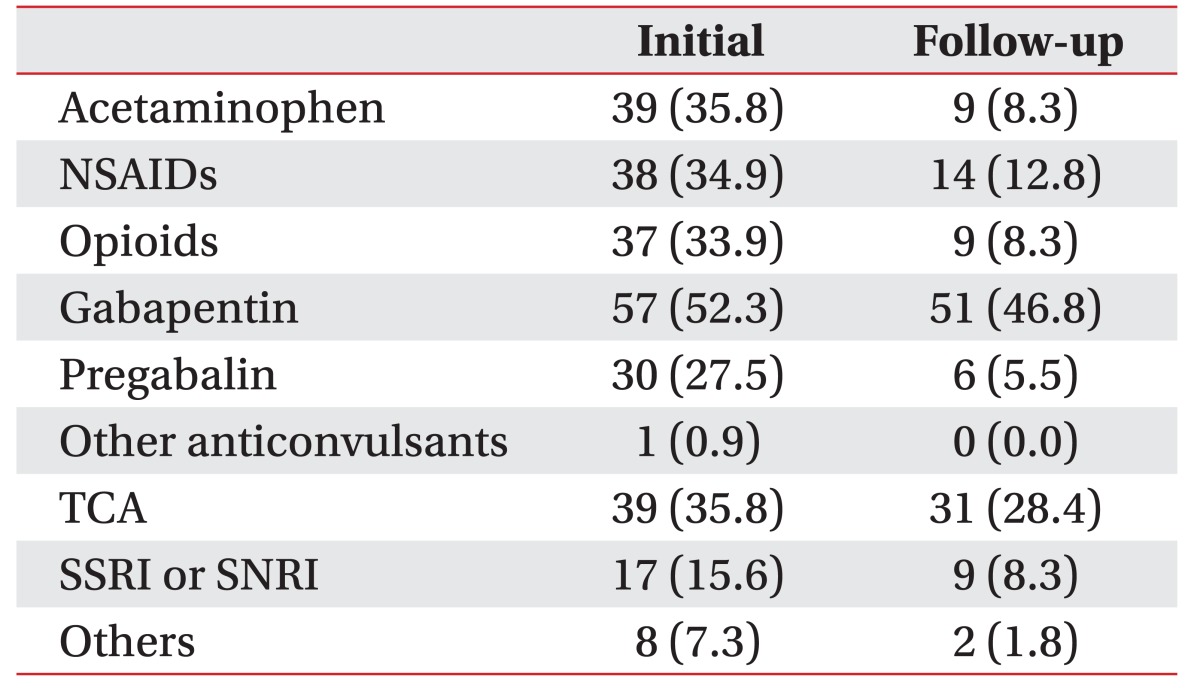
Pain medications were classified into nine categories: acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, gabapentin, pregabalin, other anticonvulsants (such as valproic acid, etc.), tricyclic antidepressants (TCAs), serotonin selective reuptake inhibitors (SSRIs) or serotonin–norepinephrine reuptake inhibitors (SNRIs), and other (such as afloqualone, etc.). Data on the number of different types of medications used at baseline and 6 weeks later (follow-up) was collected [2122].
ASIA motor subscores (sum of the upper extremity motor score and lower extremity motor score; range, 0–100) and sensory subscores (light touch and pin prick; range, 0–112, both) were assessed to evaluate physical function. The scores of the Functional Independence Measure (FIM; range, 18–126) and Spinal Cord Independence Measurement III mobility section (SCIM3; range, 0–100) were used to measure each patient's functional status.
Depressive mood was evaluated according to the Beck Depression Inventory (BDI), which determines how much depression an individual is feeling with respect to the severity of the depressive symptom [23]. This scale consists of 21 questions, and each question is scored on a scale from 0–3 (range, 0–63), with a higher score indicating more severe depression [24].
An educational pain management program was developed for patients with an SCI and neuropathic pain, and it consisted of a 6-week treatment program. All patients were assessed within 48 hours of the beginning and end of the intervention period. The educational pain management program consisted of weekly sessions, during which a physiatrist provided the following information: (i) the natural course of the SCI, (ii) SCI-related pain physiology, and (iii) types, dose, use, side effects, and costs of pain medication. After conducting the physical examination and assessing history on the initial evaluation, the patients were shown the slides containing information about (i) and (ii) for approximately 30 minutes. After identifying drugs that the patient was taking, the patients were provided information about the indications, mechanism of action, and side effects of the drug, using simple, descriptive handouts. Approximately 10 minutes, the question and response for the (i), (ii), (iii) to once a week face-to-face were performed for 6 weeks. After each educational session, the patients shared feedback with the rehabilitation specialist who determined whether the medications were effective. The medications were considered effective if the following were achieved: (1) pain reduction of at least 30% or (2) the resolution of sleep disturbance and improvement in quality of life. If these outcomes were not achieved, the medications were determined to have no significant effects. In such cases, we recommended a reduction in the use of medications, based on the Beers criteria and the previously published studies [2526]. After assessing the number of different types of medications taken at the beginning and end of the program as the secondary outcome, the patients who revealed reductions in the number of different types of medications were defined as the response group, whereas those who revealed no changes or an increase in the number of medications were defined as the non-response group.
The general characteristics or baseline data were compared between each group using the Student t-test for continuous variables, if the data was normally distributed. Associations between qualitative variables were assessed using the χ2-test or Fisher exact test. Differences between the groups regarding changes in the use of medications were measured using paired t-tests. Using the variables with either statistical or clinical significance, binary logistic regression analysis was performed to identify injury characteristics that influenced response after education. SPSS software ver. 23.0 (IBM, Armonk, NY, USA) was used to perform the statistical analyses. Statistical significance was considered when the p-value was less than 0.05.
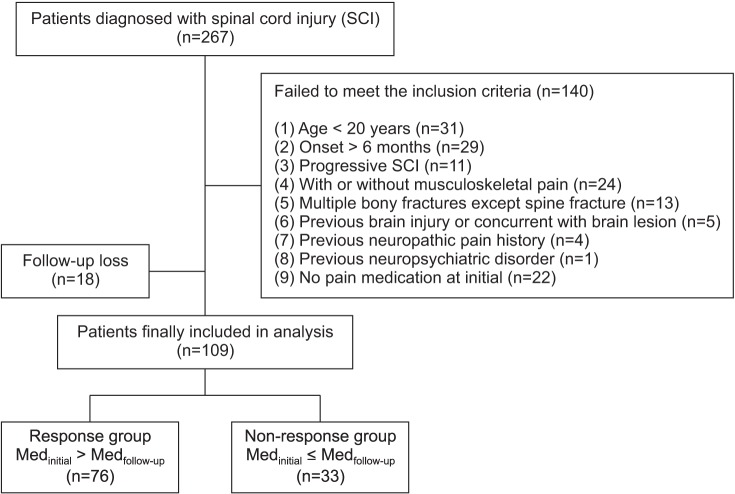
Among all patients, 267 had an SCI, of whom 109 satisfied the inclusion and exclusion criteria, as shown in Fig. 1. Seventy-five were men (68.8%), with a mean age of 50.6±13.9 years and a mean duration of pain onset of 67.1±46.4 days. Sixty-nine patients (63.3%) had cervical SCI, with an average VAS score of 4.8±2.3. Subjects took an average of 2.4±1.3 types of medications. When classifying subjects according to their responses, we classified 76 subjects into the response group and 33 into the non-response group (Fig. 1).
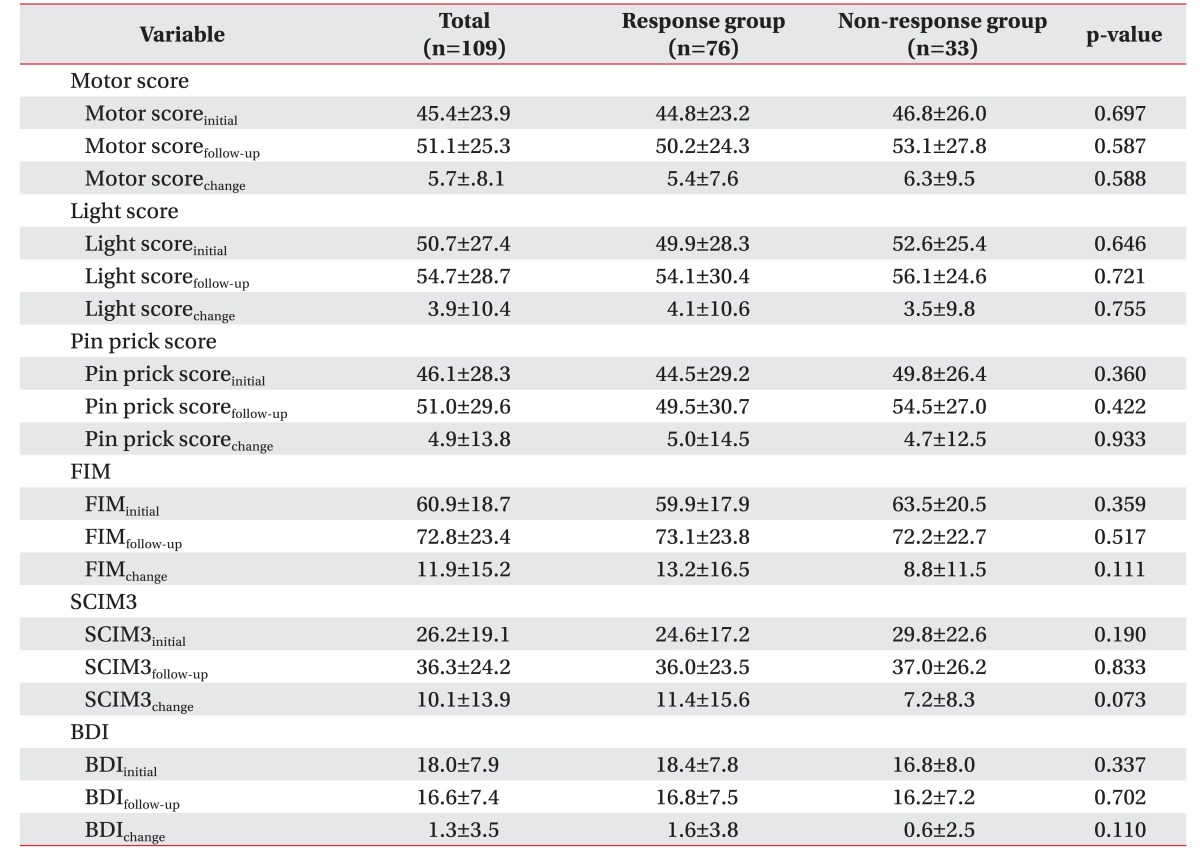
The duration of pain onset (p=0.004) revealed a statistically significant correlation with the response group. Other injury-related characteristics did not reveal significant differences in sex, age, NLI, the AIS grade, and etiology between the two groups (Table 1).
Initial VAS scores decreased at the follow-up in the response and non-response groups, with no significant differences between the two groups. There were no significant differences in responses to a reduction in medications, between the different VAS scores and pain types. An initial high number of different medications (p<0.001) and patients taking acetaminophen, NSAIDs, pregabalin, TCAs, SSRIs, or SNRIs, and other pain medications revealed statistically significant associations between the response and non-response groups (Tables 2, 3).
The physical function score, motor scores, and sensory score were not statistically significantly different between the two groups. Regarding subjects' functional level, the FIM and SCIM3 scores were not statistically significantly different between the two groups (Table 4).
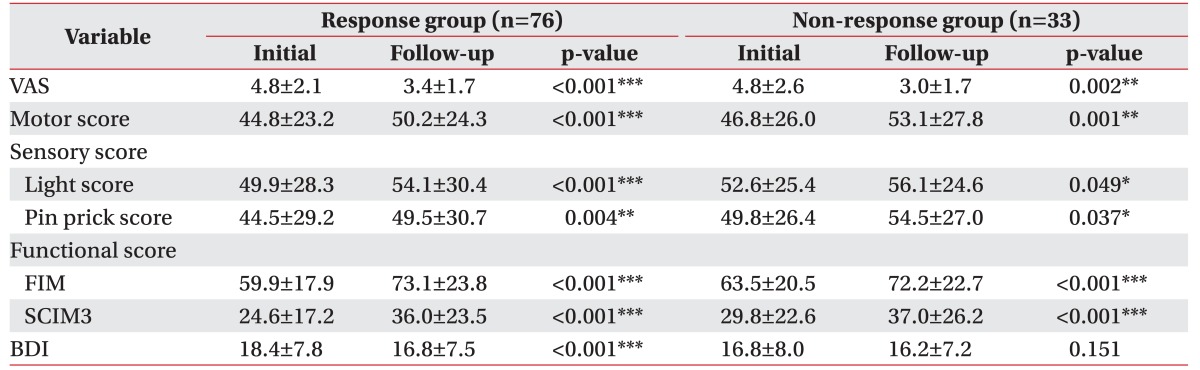
There were significant differences among all the groups for the reduction in VAS scores and increases in the motor score, sensory score, and functional score at follow-up compared to baseline measurements. The response group had a significantly lower BDI score than the non-response group (Table 5). Regression analysis revealed that there were no statistically significant correlations between the improvement in functional status or reduction in depression and the response.
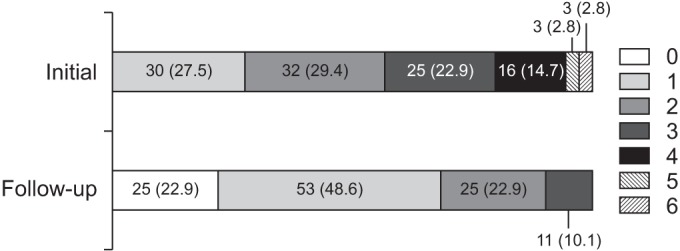
Seventy-nine (72.5%) of 109 patients took more than two types of pain medications at initial, and this number decreased to 36 (33.0%) after completing the educational pain management program (Fig. 2).
In the present study, providing education significantly reduced the number of different types of pain medications patients used. When comparing the non-response group to the response group, which is the group that revealed a reduction in medications at the follow-up, duration of pain after onset, initial number of different medications, and certain types of medication had significant effects on reducing pain medications in patients with neuropathic pain with acute and subacute SCI.
Studies on reducing pain medications in SCI are lacking. However, there are studies about the factors of prevalence of neuropathic pain [15] and epidemiology of polypharmacy [2127]. A previous study [28] reported that age at the time of injury is an important factor, associated with adaptation to the overall injury and patient's well-being. In addition, another study [121] suggested that age at the time of injury, male sex, and complete injury are associated with the onset of neuropathic pain. Therefore, we predicted that the effect of education would be significantly associated with these factors; however, in our study, there was no overall difference in the prevalence of neuropathic pain between those with neurological level of injury, sex, and age at the time of injury. For elderly or complete SCI patients, secondary health conditions are associated with diseases that increase polypharmacy [21]. In contrast, though our study differs from those that focus only on pain medications, results in the age itself are not associated with reduction of pain medication. A study conducted by Hanlon et al. [29] demonstrated that the effect of education intervention on reduction in medications differs, depending on patients' educational level and sex.
Education was found to be effective for patients with a recent onset of pain. Compared to patients in the non-response group, those in the response group had a significantly short duration of pain after injury (<60 days). A study conducted by van Gorp et al. [5] demonstrated that the time after SCI was significantly correlated with the prevalence of pain. Besides, Wand et al. [30] demonstrated that an early educational intervention provided during the acute stage showed a more long-term effect on patients. Therefore, it is important for clinicians to perform early and appropriate treatment, as it can prevent neuropathic pain from becoming unnecessarily chronic [30].
Education was effective for patients taking many different types of medications. This suggests that prescribing multiple medications, does not adequately represent patients' demands. Acetaminophen or NSAIDs, whose efficacy for neurogenic pain has barely been verified [31], were prescribed to about one-third of patients. Additionally, these medications were medications that we markedly reduced, for use through education. This suggests that neuropathic pain is incorrectly diagnosed as musculoskeletal pain, so it does not receive proper treatment. A differential diagnosis of neuropathic pain and musculoskeletal pain is difficult in patients with an SCI [32]. It is important to make an accurate diagnosis and prescribe medicine based on a detailed medical history and physical examination, and to perform regular reviews and monitoring. Appropriate treatment includes prescribing medications according to patients' symptoms and considering the cessation of medications based on regular evaluating the drugs' efficacy. If the patient is already taking multiple medications, reviewing the accuracy of the diagnosis and effect of the medications, and providing such information to the patient for feedback may result in a positive outcome.
Similar results were observed in a study [33] that conducted a face-to-face consultation with the physician; additionally, direct involvement of the physician in the intervention compared to written recommendations achieved a greater reduction in the number of medications. Furthermore, in our study, we observed that providing regular education during each medication cycle is effective for reducing polypharmacy and subjective pain intensities. Although there were no differences in the responses to education in accordance with physical states and the intensity and characteristics of pain before education, patients with reduced polypharmacy after education took a high proportion of antidepressants such as TCAs and SSRIs for their initial prescriptions, and they revealed improvement in depression at follow-up. These findings support those of several previous studies, which have suggested that depression has a significant correlation with the prevalence of pain and emotional states are associated with neuropathic pain [5]. These findings suggest that providing emotional support, would be conducive to the treatment of neuropathic pain in patients with an SCI.
This study has some limitations. First, subjects' socioeconomic statuses, including educational level were not assessed. The present study revealed that socioeconomic circumstances and family compositions affect neuropathic pain, but they may also have a crucial role, as financial hardship is particularly associated with comorbidities and pain [34]. It is proven that educational level influences the reduction in polypharmacy in elderly patients [29], and that educational level is affected by sex. Second, we could not subjectively survey patients' satisfaction after reducing their medications [14]. According to a previous study [1], 70% of patients with an SCI with pain responded that pain affected their life significantly. Hence, examining the correlation between changes in pain caused by a reduction in pain medications and enhancements in quality of life, activities of daily living, and sleep patterns would have provided more meaningful implications. Third, selection bias may have occurred. Although we reduced pain medications based on the feedback of patients, a physical examination, and the results of previous published studies [3536], the physicians' subjective opinion could potentially affect results. Fourth, further research that considers the dosage and number of different medicines taken is needed. Fifth, the long-term effects of the intervention in this study were not measured, and a comparison with a control group was not performed. Pitkala et al. [27] conducted a hospital-based study, on an intervention to reduce polypharmacy in elderly patients for 12 months; they found that the number of medications used were significantly reduced during in-hospital care monitoring, but this value reverted to the baseline value after the patients returned home. Further randomized, control studies and long-term follow-ups are needed to quantify the relationship between non-pharmacological methods and pain reduction outcomes.
Many physicians do not request patients to compile a complete list of drugs taken, or they do not review the patient's drug list regularly to evaluate medications that could be stopped [37]. Appropriate polypharmacy is not just about reducing the number of drugs, as the prescription of medication should also be appropriate to the patients' needs [37]. The present study demonstrated that rehabilitation specialists can reduce inappropriately prescribed drugs and possibly adverse drug effects, due to neuropathic pain through pharmaceutical education without adversely affecting the health-related quality of life of patients with SCI and neuropathic pain. Researchers have argued that few treatments have clearly demonstrated efficacy, particularly with the treatment of neuropathic SCI pain [38].
In conclusion, for patients with neuropathic pain, providing education would be a cost-effective way to reduce the side effects of medication and increase patients' stability while reducing pain. Hence, reducing the number of medications by effectively implementing education as an early intervention for patients and more initial medication types, may be conducive to the long-term management of patients.
ACKNOWLEDGMENTS
This study was supported by the Research Institute of Rehabilitation Medicine at Yonsei University College of Medicine.
References
1. Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury: relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004; 42:665–673. PMID: 15289801.
2. Werhagen L, Hultling C, Molander C. The prevalence of neuropathic pain after non-traumatic spinal cord lesion. Spinal Cord. 2007; 45:609–615. PMID: 17160075.

3. Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003; 103:249–257. PMID: 12791431.

4. Norrbrink Budh C, Hultling C, Lundeberg T. Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord. 2005; 43:85–95. PMID: 15570322.

5. van Gorp S, Kessels AG, Joosten EA, van Kleef M, Patijn J. Pain prevalence and its determinants after spinal cord injury: a systematic review. Eur J Pain. 2015; 19:5–14. PMID: 24824334.

6. Cuff L, Fann JR, Bombardier CH, Graves DE, Kalpakjian CZ. Depression, pain intensity, and interference in acute spinal cord injury. Top Spinal Cord Inj Rehabil. 2014; 20:32–39. PMID: 24574820.

7. Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009; 47:352–359. PMID: 19002150.

8. Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007; 23:383–391. PMID: 17515736.

9. van Leeuwen CM, Post MW, van Asbeck FW, Bongers-Janssen HM, van der Woude LH, de Groot S, et al. Life satisfaction in people with spinal cord injury during the first five years after discharge from inpatient rehabilitation. Disabil Rehabil. 2012; 34:76–83. PMID: 21870935.

10. Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010; 85(3 Suppl):S3–14.

11. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010; 9:807–819. PMID: 20650402.

12. Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008; 22:455–475. PMID: 18484790.

13. Backonja MM, Irving G, Argoff C. Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 2006; 10:34–38. PMID: 16499828.

14. Siddall PJ, Middleton JW. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006; 44:67–77. PMID: 16116488.

15. Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010; 17:1113–1e88. PMID: 20402746.

16. Kitzman P, Cecil D, Kolpek JH. The risks of polypharmacy following spinal cord injury. J Spinal Cord Med. 2017; 40:147–153. PMID: 24970339.

17. Mehta S, Orenczuk K, McIntyre A, Willems G, Wolfe DL, Hsieh JT, et al. Neuropathic pain post spinal cord injury part 1: systematic review of physical and behavioral treatment. Top Spinal Cord Inj Rehabil. 2013; 19:61–77. PMID: 23678287.

18. Meyer TJ, Van Kooten D, Marsh S, Prochazka AV. Reduction of polypharmacy by feedback to clinicians. J Gen Intern Med. 1991; 6:133–136. PMID: 2023020.

19. Widerstrom-Noga E, Biering-Sorensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, et al. The International Spinal Cord Injury Pain Basic Data Set (version 2.0). Spinal Cord. 2014; 52:282–286. PMID: 24469147.

20. Bryce TN, Biering-Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, et al. International spinal cord injury pain classification. Part I. Background and description. March 6-7, 2009. Spinal Cord. 2012; 50:413–417. PMID: 22182852.
21. Hwang M, Zebracki K, Vogel LC. Medication profile and polypharmacy in adults with pediatric-onset spinal cord injury. Spinal Cord. 2015; 53:673–678. PMID: 25896344.

22. Finnerup NB, Baastrup C. Spinal cord injury pain: mechanisms and management. Curr Pain Headache Rep. 2012; 16:207–216. PMID: 22392531.

23. Kalpakjian CZ, Bombardier CH, Schomer K, Brown PA, Johnson KL. Measuring depression in persons with spinal cord injury: a systematic review. J Spinal Cord Med. 2009; 32:6–24. PMID: 19264045.

24. Ataoglu E, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord. 2013; 51:23–26. PMID: 22547044.

25. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012; 60:616–631. PMID: 22376048.
26. Teasell RW, Mehta S, Aubut JA, Foulon B, Wolfe DL, Hsieh JT, et al. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010; 91:816–831. PMID: 20434623.

27. Pitkala KH, Strandberg TE, Tilvis RS. Is it possible to reduce polypharmacy in the elderly? A randomised, controlled trial. Drugs Aging. 2001; 18:143–149. PMID: 11346128.
28. Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001; 82:1571–1577. PMID: 11689978.
29. Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996; 100:428–437. PMID: 8610730.

30. Wand BM, Bird C, McAuley JH, Dore CJ, MacDowell M, De Souza LH. Early intervention for the management of acute low back pain: a single-blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine (Phila Pa 1976). 2004; 29:2350–2356. PMID: 15507794.
31. Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: a survey study. J Spinal Cord Med. 2006; 29:109–117. PMID: 16739554.

32. Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016; 157:1599–1606. PMID: 27115670.

33. Rollason V, Vogt N. Reduction of polypharmacy in the elderly: a systematic review of the role of the pharmacist. Drugs Aging. 2003; 20:817–832. PMID: 12964888.
34. Mahnig S, Landmann G, Stockinger L, Opsommer E. Pain assessment according to the International Spinal Cord Injury Pain classification in patients with spinal cord injury referred to a multidisciplinary pain center. Spinal Cord. 2016; 54:809–815. PMID: 26754471.

35. Tarride JE, Gordon A, Vera-Llonch M, Dukes E, Rousseau C. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther. 2006; 28:1922–1934. PMID: 17213013.

36. Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol. 2008; 64:851–858. PMID: 18607580.

37. Reddy PS, Waseemuddin M. Assessment of drug related problems in geriatrics with polypharmacy and risk measurement. Asian J Pharm Technol Innov. 2014; 2:39–58.
38. Siddall PJ, Middleton JW. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006; 44:67–77. PMID: 16116488.

Fig. 1
Flowchart of the subjects. A total 267 patients with spinal cord injury (SCI) were assessed from January 1, 2014 to December 31, 2014. Finally, 109 patients were included and they were classified into two groups according to the response.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download