This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To compare the effectiveness of extracorporeal shock wave therapy (ESWT) and trigger point injection (TPI) for the treatment of myofascial pain syndrome in the quadratus lumborum.
Methods
In a retrospective study at our institute, 30 patients with myofascial pain syndrome in the quadratus lumborum were assigned to ESWT or TPI groups. We assessed ESWT and TPI treatment according to their affects on pain relief and disability improvement. The outcome measures for the pain assessment were a visual analogue scale score and pain pressure threshold. The outcome measures for the disability assessment were Oswestry Disability Index, Roles and Maudsley, and Quebec Back Pain Disability Scale scores.
Results
Both groups demonstrated statistically significant improvements in pain and disability measures after treatment. However, in comparing the treatments, we found ESWT to be more effective than TPI for pain relief. There were no statistically significant differences between the groups with respect to disability.
Conclusion
Compared to TPI, ESWT showed superior results for pain relief. Thus, we consider ESWT as an effective treatment for myofascial pain syndrome in the quadratus lumborum.
Go to :

Keywords: Extracorporeal shock wave therapy, Myofascial pain syndromes, Trigger point injection, Quadratus lumborum
INTRODUCTION
Myofascial pain syndrome (MPS) of the spinal stabilizer muscles is one of the most frequent causes of chronic low back pain. However, MPS is often overlooked. Among the spinal stabilizer muscles, the quadratus lumborum (QL) is frequently a trigger point and location of referred low back pain [
1]. Overuse and strain of the QL is one of the major causes of chronic pain in the lower back.
MPS is characterized by symptoms that include localized muscular tenderness, myofascial trigger points, a palpable intramuscular taut band, and a muscular twitching response [
2]. MPS is a common cause of pain and dysfunction in the musculoskeletal system and accounts for 20% to 95% of cases with musculoskeletal pain presenting at outpatient clinics and pain management centers [
3]. The primary goal of managing MPS is to break the vicious cycle of pain through the elimination of trigger points [
4]. There are various treatments for the elimination of myofascial trigger points, including trigger point injection (TPI) [
5], ischemic compression, stretching, massage, and treatment modalities, including ultrasound and transcutaneous electrical nerve stimulation. A variety of treatments exist, but the most effective treatment for MPS is still under debate.
Recently, extracorporeal shock wave therapy (ESWT) has advanced as an alternative treatment for MPS in patients with symptoms recalcitrant to traditional conservative treatment [
6]. ESWT is defined as a sequence of single sonic pulses characterized by high peak pressure – 100 MP, a fast onset of pressure (<10 ns), and short duration (10 µs). ESWT is conveyed by an appropriate generator to a specific target area with an energy density in the range of 0.003–0.890 mJ/mm
2 [
7]. The transduction of an ESWT acoustic shock wave signal into a biological signal results in cell proliferation and/or differentiation via a mechano-transduction process [
8]. Advantages of ESWT include its non-invasiveness and minimal side effects. As such, research on ESWT has recently increased to explore its effectiveness, particularly for MPS in the upper trapezius [
9]. Thus far, research ESWT's effectiveness is limited as to other areas, including the QL.
Therefore, the aim of this study was to evaluate the clinical efficacy of ESWT for MPS in the QL. Our evaluation compared pain and disability indices between two groups of subjects: patients who treated with ESWT, and patients who treated with TPI.
Go to :

MATERIALS AND METHODS
Subjects
We obtained approval for this study from the ethics committee of Gangnam Severance Hospital. This retrospective study enrolled 30 patients who were hospitalized at our institute between April 2015 and June 2016. Patients had a single diagnosis of MPS in the QL for lower back pain that persisted for more than 3 months. We conducted detailed medical examinations, administered by interviews and physical examinations. Based on the diagnosis standard presented by Simons and Mense [
10] and Vecchiet et al. [
11], we diagnosed the subjects as having MPS when they exhibited the following: pain at local sites of QL muscle; short taut bands formed; pain when the QL muscles were pressed; and pain trigger points, causing referred pain at relatively accurate spots around the QL muscles. We detected trigger points by using both thumbs while subjects lay in prone postion.
To improve the valid inclusion criteria, study subjects were included when all described conditions were satisfied. Differential diagnosis was also evaluated by other examinations. These examinations included L-spine MRI, EMG, and simple X-ray for differentiating other pathologies, such as spinal stenosis, spinal tumor, herniated nucleus pulposus, spondylolisthesis, spinal instability, and spinal anomaly under medical imaging inspection, and lumbosacral, radiculopathy, and myelopathy.
We excluded subjects with the following: a prior history of receiving ESWT or lumbar spine surgery; neurological deficits involving the lower extremities; cardiovascular disease; inflammatory arthritis; local infection; malignancy; cardiac arrhythmia; cardiac pacemaker; and pregnancy.
For patients diagnosed with MPS, we confirmed their sex, age, time of occurrence of the lesion, area of the lesion, concomitant diseases, and presence of any preceding injury. We then allowed the patients to select between ESWT or TPI as their treatment method after we explained our study's purposes. Of the 30 patients, 15 received a TPI and 15 received ESWT. All patients underwent conservative treatment that included analgesics, rest, and therapeutic exercises.
Primary outcome measure: pain evaluation
The primary outcome measure is an assessment of pain using two methods: (1) a visual analogue scale (VAS) and (2) measurement of the pressure pain threshold (PPT).
We used the VAS to estimate pain intensity on a scale of 0 to 10. A score of 10 indicated maximal pain and 0 indicated no pain.
We measured PPT as patients lay in a prone position. We used ultrasonography to mark the location for the measurement at the mid-point of the QL muscle belly. To do this, we vertically applied a digital algometer (OE-220, ITO, Tokyo, Japan) to the surface of the tender point, and the subjects were instructed to say “stop” when they felt pain. That moment was measured in units of kg/cm
2. We repeated this procedure three times at 30-second intervals, and recorded the average value as the PPT. Using a digital algometer, we evaluated the criterion level of pain or discomfortness at VAS 5 level, and confirmed the PPT of a tender point when there was a difference of more than 2 kg/cm
2, compared to a non-tender point. PPT was recoded in the case of both described conditions. [
12]
Secondary outcome measure: disability evaluation
The secondary outcome measure assesses disability using three methods: the Oswestry Disability Index (ODI); the Roles and Maudsley (RM) score; and the Quebec Back Pain Disability Scale (QBS). The ODI is a self-administered measuring tool, conducted by checking the subject's level of pain during nine different activities: personal hygiene, lifting objects, walking, sitting, standing, sleeping, social activity, traveling, and ambulation. Higher scores indicate a greater dysfunctional status resulting from the lower back pain [
13]. The RM score is a functional assessment of pain during daily life activities, scored as excellent, good, fair, or poor [
14]. The QBS is a condition-specific questionnaire designed to measure the level of functional disability for patients with lower back pain. The QBS measures for 20 daily activities under six overall categories.
Treatment
To identify the QL muscle, we used the Accuvix V10 (Samsung Medison, Seoul, Korea) with a linear transducer at frequencies of 5–13 MHz. Patients were in the prone position during the examination.
For patients who opted for ESWT, we used a Dornier AR2 with smart focus technology (MedTech, Munchen, Germany). We applied the device at the tender point of the QL muscle belly, with 2,000 shock waves applied at each session at an intensity of 0.085–0.148 mJ/mm2. We repeated this procedure a total of three times, at 3-day intervals.
For TPI patients, we similarly administered the TPI three times at the tender point of the QL at 3-day intervals. For this procedure, we used a digital algometer for localizing the tender point by measuring pain threshold.
We conducted ESWT and TPI in a ultrasound-guided fashion to diminish bias resulting from technical errors. We conducted a gross observation of the ultrasound to check for twitch response.
Assessment
We assessed clinical outcomes three times: before the initial treatment (pre-treatment assessment), immediately after the third treatment (post-treatment assessment), and 1 month after treatment at the outpatient clinic (follow-up assessment). To compare the treatment efficacies, we recorded differences seen in the post-treatment and follow-up assessment, compared to the pre-treatment assessment (
Table 1). We monitored adverse effects both before and after the application of the ESWT or TPI.
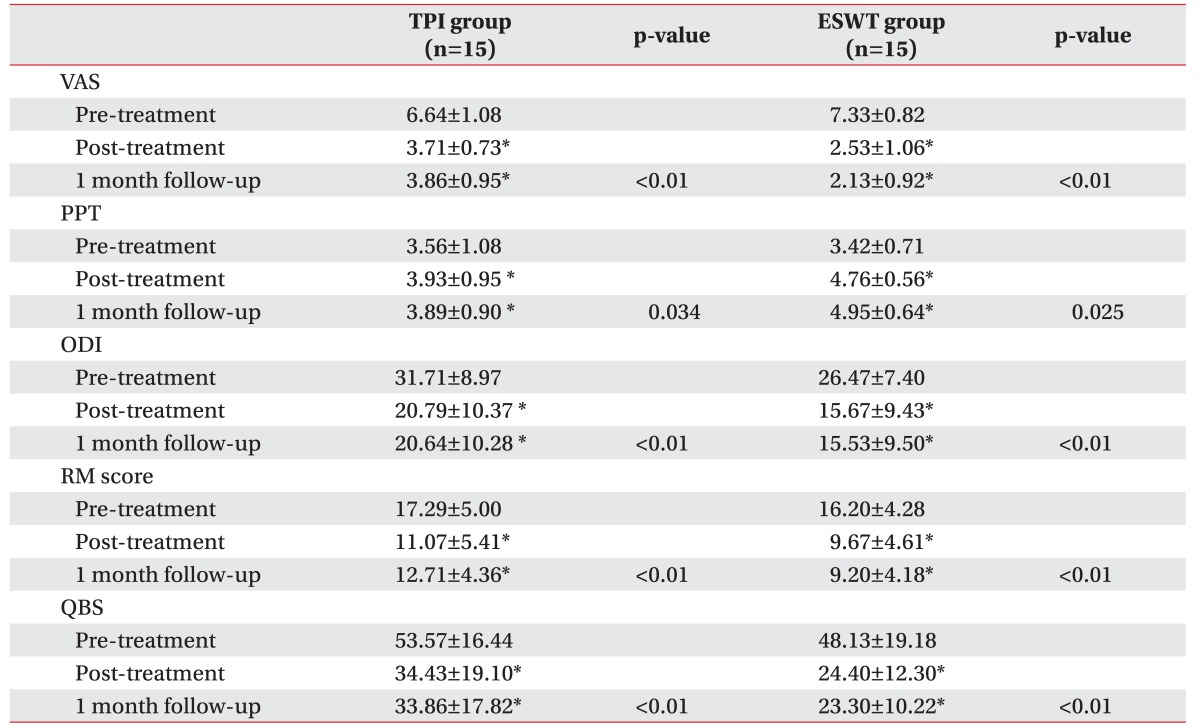
Table 1
The change of clinical outcomes of ESWT and TPI groups

Statistical analyses
We used general linear modeling as our main analytic approach. A univariate repeated-measures analysis of variance (ANOVA) was conducted for each of the three assessment times for each outcome measure (VAS, PPT, ODI, RM score, and QBS) and treatment group (ESWT group vs. TPI group). This approach permitted the modeling of both individual and sets of outcome measures, as a function of treatment effects (between subjects) and time of assessment (within subjects). Hence, the interaction effect of treatment × time was of greatest interest. We also applied an independent t-test to compare the differences between treatment groups.
All tests of statistical significance were interpreted with a criterion of p<0.05. We performed our statistical analyses using SPSS release 12.0.1 for Windows (SPSS Inc., Chicago, IL, USA).
Go to :

RESULTS
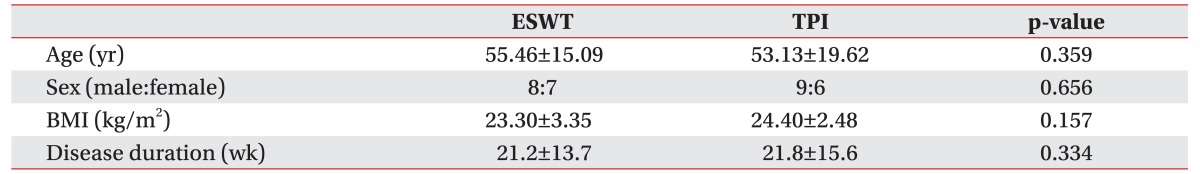
The study included 30 patients (17 men and 13 women). Of the 15 subjects in the ESWT group, 8 were men and seven were women. In the TPI group, 9 were men and 6 were women. We observed no statistical differences between the two groups in terms of age, sex, body mass index, disease duration, and pre-treatment clinical outcomes (
Table 2).
Table 2
Demographic comparison between ESWT and TPI groups

In comparing the scores measured pre-treatment, the clinical outcome assessment scores taken post-treatment and at the 1-month follow-up exam showed statistically significant differences in both groups, with decreases in the VAS, ODI, RM, and QBS scores, and an increase in the PPT (
Table 1). Moreover, the clinical outcome scores between post-treatment and 1-month follow-up showed no significant statistical difference in each study group (
Table 1). These results suggest that both ESWT and TPI are useful treatments to improve pain and relieve disability in MPS patients with lower back pain.
We found no statistically significant difference (p>0.05) between VAS and PPT in pre-treatment by an independent t-test. However, we found significant differences between pre-treatment and post-treatment and pretreatment and the 1-month follow-up (p<0.05) (
Fig. 1A, 1B). There was a steeper difference in pain reduction in ESWT, compared to TPI.
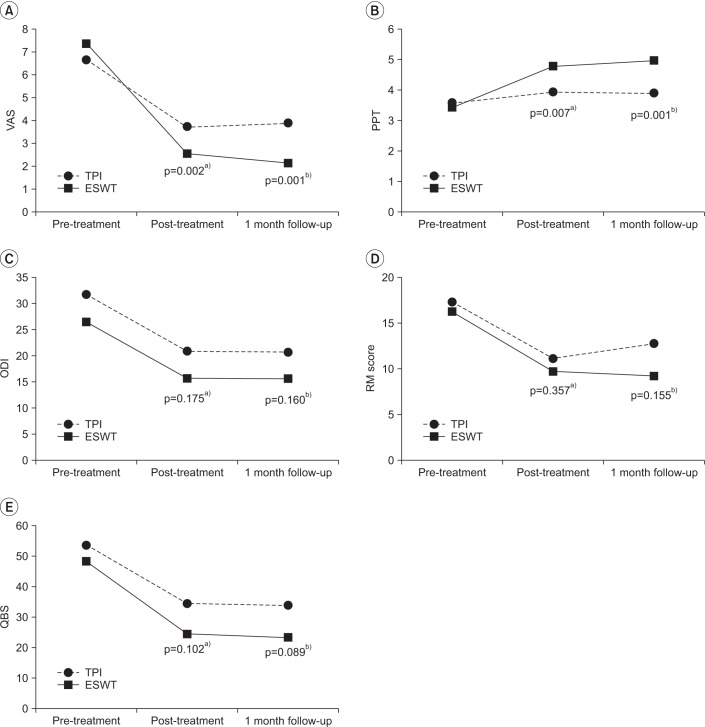 | Fig. 1Comparison of primary and secondary outcome measure scores in the ESWT and TPI groups. The squares and solid lines show results for the ESWT group, while the rounds and dotted lines represent results for the TPI group. ESWT, extracorporeal shock wave therapy; TPI, trigger point injection; VAS, visual analogue scale; PPT, pain pressure threshold; ODI, Oswestry Disability Index; RM score, Roles and Maudsley Score; QBS, Quebec Back Pain Disability Scale. Significant differences a)between pre-treatment and post-treatment and b)between pretreatment and the 1 month follow-up.
|
However, there were no statistically significant differences between ESWT and TPI in the secondary disability indexes of ODI, RM score, and QBS when comparing immediate post-treatment and 1 month after (p>0.05) (
Fig. 1C–1E).
There were no serious adverse events, such as intolerable pain or subcutaneous infection, after treatment in either group.
Go to :

DISCUSSION
Our results showed that all three sessions of ESWT and TPI significantly reduced pain and improved the quality of life of patients with MPS in the QL, with no observable adverse effects. The comparison between the treatment groups showed there was a significantly greater pain reduction in the ESWT group, but no significant difference in the disability evaluation parameters. These findings suggest that ESWT is more effective than TPI for treating pain in patients with MPS in QL.
The mechanism by which ESWT reduces MPS remains uncertain. MPS is a state in which stimulation of a trigger point in a muscle can result in referred pain. It is accompanied by deep pain that occurs in a fascia area connecting to various muscle groups and bone. It is therefore important to eliminate the trigger point. Many researchers have sought to understand the effect of the charge applied in ESWT on the elimination of this trigger point. For example, it has been reported that applying ESWT to the lesion stimulates an increase in blood flow and the reformation of blood vessels [
15]. In turn, this application stimulates a curative process in the muscle, tendon, and surrounding tissues/bones, leading to reactivation. A further explanation may be that when the A delta receptor, which exhibits rapid neuro-stimulator transmission, is stimulated, this suppresses C fiber stimulation. The C fiber stimulation has a slow neuro-stimulator transmission and is long lasting, and its suppression thereby blocks nerve conduction [
16].
Our results differed from studies evaluating the use of ESWT for MPS in the upper trapezius muscle. Those studies showed no significant difference, whereas we found a significantly greater reduction in pain in the ESWT group compared to the TPI group. The different results may be due to the different locations of the QL and upper trapezius muscles. The QL is more deeply located than the upper trapezius. Thus, we had a limitation in targeting TPI at the exact depth, although we applied ultrasound to increase the preciseness of the procedure. In addition, a previous study used a lower energy of 0.056 mJ/mm
2 applied at 1,000 impulses to the taut band, whereas our study used a higher energy of 0.085–0.148 mJ/mm
2 at 2,000 impulses. As ESWT has a dose-dependent effect [
17], the use of different intensities and impulses may have influenced the treatment results. Finally, TPI is not always capable of thoroughouly addressing diffusely-located multiple taut bands. In such cases, ESWT is an appropriate treatment option, with broader coverage and without post-injection soreness.
Similar to the previous studies, we found no significant differences when comparing the disability evaluation. This may be due to the relatively short follow-up period. Any further study may need to include a longer follow-up period. Additionally, disability may result from factors other than pain itself. It is also difficult to completely control the subjects' daily activities.
Based on our results, we can recommend ESWT as an effective treatment. It has many advantages, including non-invasiveness, less pain and complications compared to TPI, scarless cosmetic advantages, and no potential risks for infection or allergic reactions [
18].
This study has three main limitations. First, this study was a retrospective study not a randomized controlled study. Therefore, subjects' preferences towards treatment method may have cause biased results. A future randomized control study should be attempted. Second, our sample size was relatively small with a short-term follow-up period. To increase compliance with ESWT, we limited our study subjects only to inpatients, and it was difficult to complete long-term follow-up after the 1 month of post-treatment from the hospital. Because we only examined the immediate outcomes, we do not have the data to establish long-term effects. Third, this study lacks a placebo control group. However, we considered it unethical to withhold treatment from patients with pain and disability during the study period.
In conclusion, ESWT for MPS in the QL resulted in a significantly greater reduction in pain compared to TPI, demonstrating the possibility of ESWT being an effective, non-invasive treatment for patients with MPS in the QL.
Go to :

ACKNOWLEDGMENTS
This study was supported by 2016 fund from the Je Won Research Foundation.
Go to :

Notes
Go to :

References
1. Simons DG, Travell J. Myofascial trigger points, a possible explanation. Pain. 1981; 10:106–109. PMID:
7232007.
2. Novikova LB, Akopyan AP. Myofascial pain syndrome. Zh Nevrol Psikhiatr Im S S Korsakova. 2015; 115:21–24.

3. Chen CK, Nizar AJ. Myofascial pain syndrome in chronic back pain patients. Korean J Pain. 2011; 24:100–104. PMID:
21716607.

4. Jafri MS. Mechanisms of myofascial pain. Int Sch Res Notices. 2014; 2014.

5. Sabatke S, Scola RH, Paiva ES, Kowacs PA. Injection of trigger points in the temporal muscles of patients with miofascial syndrome. Arq Neuropsiquiatr. 2015; 73:861–866. PMID:
26465403.
6. Borg-Stein J, Iaccarino MA. Myofascial pain syndrome treatments. Phys Med Rehabil Clin N Am. 2014; 25:357–374. PMID:
24787338.

7. Moon SW, Kim JH, Jung MJ, Son S, Lee JH, Shin H, et al. The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann Rehabil Med. 2013; 37:461–470. PMID:
24020026.

8. Shrivastava SK, Kailash . Shock wave treatment in medicine. J Biosci. 2005; 30:269–275. PMID:
15933416.

9. Ji HM, Kim HJ, Han SJ. Extracorporeal shock wave therapy in myofascial pain syndrome of upper trapezius. Ann Rehabil Med. 2012; 36:675–680. PMID:
23185732.

10. Simons DG, Mense S. Diagnosis and therapy of myofascial trigger points. Schmerz. 2003; 17:419–424. PMID:
14648314.
11. Vecchiet L, Giamberardino MA, Saggini R. Myofascial pain syndromes: clinical and pathophysiological aspects. Clin J Pain. 1991; 7(Suppl 1):S16–S22. PMID:
1810517.
12. Delaney GA, McKee AC. Inter- and intra-rater reliability of the pressure threshold meter in measurement of myofascial trigger point sensitivity. Am J Phys Med Rehabil. 1993; 72:136–139. PMID:
8512674.

13. Park SW, Shin YS, Kim HJ, Lee JH, Shin JS, Ha IH. The dischargeable cut-off score of Oswestry Disability Index (ODI) in the inpatient care for low back pain with disability. Eur Spine J. 2014; 23:2090–2096. PMID:
25099873.

14. Mousavi SJ, Parnianpour M, Mehdian H, Montazeri A, Mobini B. The Oswestry Disability Index, the Roland-Morris Disability Questionnaire, and the Quebec Back Pain Disability Scale: translation and validation studies of the Iranian versions. Spine (Phila Pa 1976). 2006; 31:E454–E459. PMID:
16778675.

15. Hammer DS, Rupp S, Ensslin S, Kohn D, Seil R. Extracorporal shock wave therapy in patients with tennis elbow and painful heel. Arch Orthop Trauma Surg. 2000; 120:304–307. PMID:
10853901.

16. Ludwig J, Lauber S, Lauber HJ, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res. 2001; 119–126.

17. Zhang X, Yan X, Wang C, Tang T, Chai Y. The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J Surg Res. 2014; 186:484–492. PMID:
24035231.

18. Furia JP. Safety and efficacy of extracorporeal shock wave therapy for chronic lateral epicondylitis. Am J Orthop (Belle Mead NJ). 2005; 34:13–19. PMID:
15707134.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download