Abstract
Objective
To evaluate characteristics of the postural instability in patients with stroke and to present a prediction model of post-stroke falls.
Methods
Patients with a first-ever stroke who had been evaluated by the Balance Master (BM) at post-stroke 3 months (±1 month) between August 2011 and December 2015 were enrolled. Parameters for the postural instability, such as the weight bearing asymmetry (WBA) and postural sway velocity (PSV), were obtained. The fall events in daily lives were assessed via structured telephone interview with a fall related questionnaire.
Results
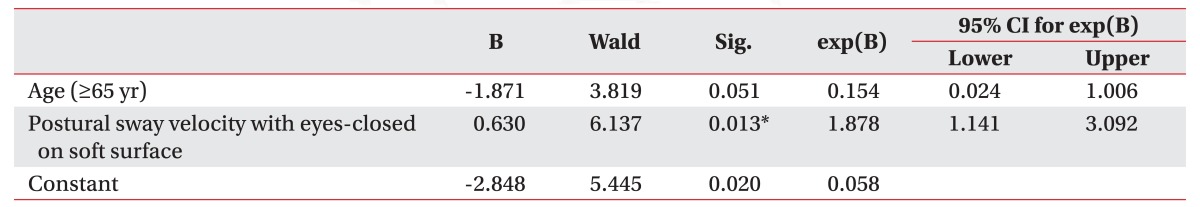
A total of 71 patients (45 men; 45 with ischemic stroke) were enrolled in this study. All subjects underwent BM evaluation at 3.03±0.40 months after stroke. The mean WBA was 17.18%±13.10% and mean PSV (measured as °/s) were noted as 0.66±0.37 (eyes-open on firm surface), 0.89±0.75 (eyes-closed on firm surface), 1.45±1.09 (eyes-open on soft surface), and 3.10±1.76 (eyes-closed on soft surface). A prediction model of post-stroke falls was drawn by multiple logistic regression analysis as follows: Risk of post-stroke falls = -2.848 + 1.878 x (PSVECSS) + 0.154 x (age=1 if age≥65; age=0 if age<65).
Stroke is one of the major causes of permanent disability [1]. A majority of stroke survivors suffer from a combination of sensory, motor, cognitive and emotional impairments, thus restricting their capacity to perform activities of daily living (ADL) [2]. Of all possible sensorimotor consequences of stroke, impaired postural control, which results in postural instability, has a significant impact on gait and independence in ADL [3456]. Postural instability is defined as the inability to control the center of mass of one's body within a given base of support [78]. The ultimate consequence of postural instability is falling [9]. Falls are very common among people who have experienced stroke with an incidence of 50% to 70% [10]. Post-stroke falls lead to severe health complications such as hip fractures, and fear of falling discourages physical activity and social participation [11]. Therefore, fall is an important clinical concern in individuals with stroke both during rehabilitation and thereafter [121314].
Various measures have been adopted to assess the post-stroke postural instability, such as the Berg Balance Scale (BBS) [1516], the weight-bearing asymmetry (WBA) [17], the force and timing asymmetry [18], the spatio-temporal and kinematic asymmetry ratio [19], electromyography [20], and the postural sway at standing [2122]. Of these, the WBA and postural sway velocity (PSV) are considered easy-to-use to assess the post-stroke postural instability quantitatively [9]. The WBA is defined as a function of body weight, expressed as the loading ratio between the affected and unaffected leg [23]. The assessment of postural sway is largely classified into two categories: the PSV [2122] and postural sway amplitude (PSA) [242526]. The PSV is more reliable and sensitive than the PSA [92728]. The WBA is the static characteristic of standing balance related to the body position, and PSV is the dynamic characteristic of standing balance related to the body movement [29]. The WBA having more weight on the non-paretic leg and increased PSV, are characteristics of the upright stance in patients with post-stroke hemiplegia [2430]. Although WBA significantly improves within the first 4 weeks of rehabilitation therapy, some degree of asymmetry persists [2931]. Also, although the PSV decreases during the first 12 weeks of rehabilitation, it remains higher than the reference value [29].
We aimed to show the characteristics of postural instability and to predict post-stroke falls by quantitative balance parameters.
Patients who were admitted for rehabilitation therapy following a first-ever stroke in the Boramae Medical Center between August 2011 and December 2015 were screened. Inclusion criteria were a patient (1) who was ≥19 years old, (2) who could stand without any aid or manual contact for at least 30 seconds, and (3) who was evaluated by the Balance Master (BM) system (NeuroCom International Inc., Clackamas, OR, USA) at post-stroke 3 months (±1 month). Exclusion criteria included a patient (1) whose muscle strength of the hip and knee extensors in the affected side was less than 3 (as Medical Research Council manual muscle testing grading), (2) with other disorders which could affect sensorimotor functions, such as Parkinson's disease, multiple sclerosis, lumbosacral radiculopathy, peripheral neuropathy, musculoskeletal problems involving the lower extremity, and visual or vestibular dysfunction, (3) whose Functional Ambulation Categories (FAC) was lower than 4, (4) with visuospatial hemineglect, anosognosia, apraxia, or aphasia disabling the relevant assessment, (5) whose Korean-Mini Mental State Examination (K-MMSE) score was lower than 27, (6) with psychiatric problems, and (7) with any history of fall within 1 year prior to stroke.
All demographic and stroke-related data were obtained, including age, sex, duration after stroke, type of stroke (ischemic or hemorrhagic), location of lesion (supratentorial or infratentorial), muscle strength of bilateral hip and knee extensors, and the presence of ataxia. Score of Fugl-Meyer Assessment (FMA) of the affected lower extremity, BBS, and Korean version of Modified Barthel Index (K-MBI) were also retrieved. All clinical evaluationas were performed on the same day as the BM test.
The BM system measured the WBA and the PSV. The BM test verifies the dynamics of postural instability by sensing loaded gravity force via the dual-force platform placed under bilateral feet. It includes a set of quantitative measurement protocols: weight-bearing squat (WBS), modified clinical test of sensory interaction on balance (mCTSIB), unilateral stance, limits of stability, rhythmic weight shift, sit to stand, tandem walk, step/quick turn, step up/over, and forward lunge. It has little inter-rater variability, and is known as valid and reliable in individuals with stroke [3233].
Among the subdomain of the BM test, the results of WBS and mCTSIB were selected to analyze the WBA and the PSV. For WBS test, subjects were instructed to bear weight on both feet as even as possible, while standing erect with extended knees. The WBA was formulated as follows: with values ranging from 0 (symmetric weight-bearing on both sides) to 100 (weight-bearing only on the unaffected side).
The mCTSIB test quantifies postural control under various sensory conditions. The support surface (firm or foam) and the visual input (eyes open or eyes closed) are selected to alter the level of somatosensory demand or visual compensation [26], simulating various conditions which the patients frequently encounter in daily activities. The mCTSIB test is known to have excellent intra-rater reliability in stroke patients [34]. The mCTSIB protocol consists of three 30-second trials for each of the 4 test conditions, and each test is terminated when a subject moves his feet. The result of mCTSIB is presented as the mean of center of gravity (COG) sway velocity under each condition, which is the ratio of the COG sway angle (expressed in degrees) to the time of the trial (expressed in seconds). The mean COG sway velocity was calculated and designated as the PSV.
At 12.47±7.29 months after stroke, information about balance-related function and falls in daily lives were obtained via structured telephone interviews using a fall-related questionnaire. The questionnaire covered FAC, number of falls or near-falls within recent one month, fear of fall, mode of transfer, and type of residence. Falls were defined as events resulting in a person coming to rest accidentally or inadvertently on the ground, and near-falls were defined as situations in which a person anticipates a fall is imminent, but avoids it by a compensatory movement. Only falls or near-falls which occurred in the fully awakened state were counted. If a subject had at least one event of fall or near-fall, the subject was classified as the faller group.
This study was approved by the Institutional Review Board of Boramae Medical Center, was conducted according to the declaration of Helsinki, and followed the Good Clinical Practice guidelines.
Statistical analysis was done by the SPSS ver. 20.0 (IBM, Armonk, NY, USA). The Shapiro-Wilk normality test was performed for ensuring the normality of data distribution such as age, interval between onset of stroke and the BM evaluation, interval between onset of stroke and telephone interview, the WBA, and PSV. To compare the variables between groups (supratentorial versus infratentorial lesion and fallers versus non-fallers) for the univariable analysis, we used the Student t-test for age, interval between onset of stroke and the BM evaluation, interval between onset of stroke and telephone interview, and the WBA; Fisher exact test for sex; and Wilcoxon rank sum test for BBS, K-MBI, FMA, K-MMSE, and the PSV. To find out the predictors of post-stroke falls, the multivariable logistic regression with backward elimination was applied by using the package ‘MASS’ of R software ver. 3.3.1 (http://www.r-project.org).
For the prediction ability of variables for post-stroke falls, the receiver operating characteristics (ROC) curve was drawn, and the area under the curve (AUC) was calculated using the package ‘pROC’ in the R program with 2000 stratified bootstrap replicates [35]. The cut-off value was calculated based on the Youden index:
For all tests, the statistical significance was set at p<0.05.
A total of 231 patients with a first-ever stroke were screened. Among them, 71 patients (45 men and 26 women; 45 with ischemic stroke and 26 with hemorrhagic stroke) were included in this study (Table 1). The mean age at the BM test was 59.15±13.27 years (range, 25–82 years). Only one patient with an infratentorial lesion had clinically evident ataxia.
The BM test was performed 3.03±0.40 months (range, 2.00–3.83 months) after stroke. The mean WBA was 17.18%±13.10% (range, 0.0%–52.0%). The PSVs in their respective test conditions (presented as °/s) were 0.66±0.37 (range, 0.2–2.1) of eyes-open on firm surface (PSVEOFS); 0.89±0.75 (range, 0.3–6.0) of eyes-closed on firm surface (PSVECFS); 1.45±1.09 (range, 0.5–6.0) of eyes-open on soft surface (PSVEOSS); and 3.10±1.76 (range, 1.1–6.0) of eyes-closed on soft surface (PSVECSS) (Table 1). The lesion location (supratentorial or infratentorial) did not affect the BBS, nor the WBA and PSV (Table 2).
Among subjects, 53 patients completed the telephone interview. The mean interval between the onset of stroke and telephone interview was 12.47±7.29 months (range, 3.63–29.10 months). The information of two subjects who had a second stroke before the telephone interview were excluded from the analysis. Mean age was 59.80±14.20 years, and mean value of FAC was 4.96±1.31 at the time of the telephone interview.
Twelve subjects (23.5%) reported falls within recent one month (Table 3). Fallers were significantly older than non-fallers (fallers, 67.50±11.65 years; non-fallers, 57.44±14.20 years; p=0.029) and had significantly lower K-MBI at post-stroke 3 months (fallers, 77.50±14.23; non-fallers, 86.56±13.68; p=0.034) (Table 3). However, there was no significant difference between fallers and non-fallers in the FMA score of the affected lower extremity, the BBS, and the K-MMSE score.
Although the WBA was similar between fallers and non-fallers, there were significant differences in the PSVs as follows: 0.72±0.22 (fallers), 0.59±0.34 (non-fallers), p=0.044 in PSVEOFS; 2.06±1.33 (fallers), 1.35±1.09 (non-fallers), p=0.023 in PSVEOSS; and 4.98±1.68 (fallers), 2.76±1.60 (non-fallers), p=0.002 in PSVECSS (Table 3). Non-fallers had a significantly higher FAC (5.26±1.21, p<0.001) at the time of telephone interview, as compared to fallers (4.00±1.21).
Multivariable logistic regression analysis was performed by backward elimination with age at the onset of stroke (≥65 or <65 years), score of the K-MBI, the PSVEOFS, the PSVEOSS, and the PSVECSS. A prediction model of post-stroke falls was drawn with age at onset and the PSVECSS (Nagelkerke R2=0.454, p=0.013) (Table 4). The equation arrived at was as follows:
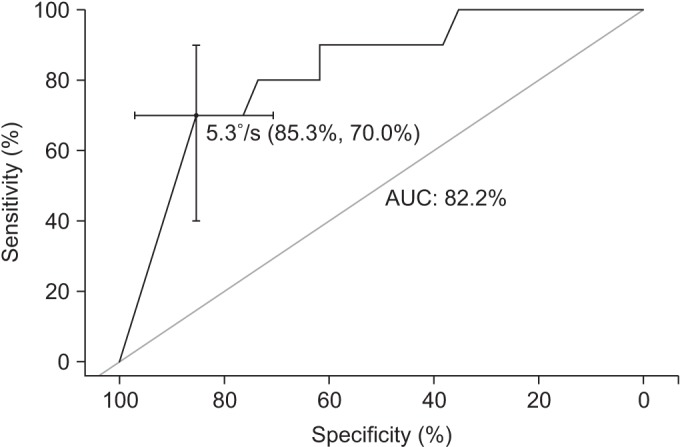
The ROC curve using the PSVECSS was also obtained (Fig. 1). The cut-off value of the PSVECSS was 5.30°/s, with a sensitivity of 70.0% (confidence interval, 40–100) and specificity of 85.3% (confidence interval, 73.53–97.06), and the AUC was 0.822.
Few trials have aimed to predict post-stroke falls. A retrospective case-control study showed that near-falls in hospital and upper limb function at the time of discharge could predict post-stroke falls in the first 12 months following discharge to the community [36]. Another study implemented a prediction model for post-stroke falls using the value of FAC, use of walking aid, time for star cancellation task, and grip strength [37]. A prospective observational study suggested that a history of falling in hospital or during rehabilitation therapy combined with poor balance (BBS or step test score) predicted the recurrent post-stroke falls [10]. However, all these studies used qualitative traits of postural instability.
There have been very few studies which investigated the correlation between results of the quantitative balance assessment and post-stroke falls [2438]. In a retrospective case-control study that analyzed patients with a mean post-stroke duration of about 12 weeks, the frequency of fall correlated with the postural sway, but not with the WBA [24]. However, there was considerable heterogeneity of post-stroke duration (3–27 weeks). Another prospective study demonstrated that the postural sway predicted falls, but subjects of this study were healthy community-dwellers [39]. To date, no study has investigated the prediction model of post-stroke falls using the quantitative parameters.
In this study, we demonstrated the static and dynamic characteristics of standing balance of individuals with stroke. The WBA in this study was similar to the value reported in a previous study of stroke patients [21]. The WBA was significantly greater compared to the normative value of the respective age groups: -0.6±3.1 (20–39 years), -1.4±3.1 (40–59 years), -1.3±3.6 (60–69 years), and -1.2±4.3 (70–79 years) (all p<0.001, all the values are presented in ‘Clinical Operation Guide' by NeuroCom International Inc.). The PSV in the respective four conditions were also significantly greater than the normative values of healthy individuals, for the respective age groups 20–39, 40–59, 60–69, and 70–79 years; PSVEOFS 0.26±0.07, 0.27±0.12, 0.28±0.12, and 0.37±0.18; PSVECFS 0.29±0.10, 0.33±0.13, 0.31±0.11, and 0.41±0.14; PSVEOSS 0.53±0.13, 0.63±0.17, 0.69±0.15, and 0.84±0.16; PSVECSS 1.27±0.39, 1.61±0.45, 1.60±0.52, and 2.05±0.64 (all p<0.001, all the values are presented in ‘Clinical Operation Guide’ by NeuroCom International Inc.).
No significant difference was observed in the static and dynamic characteristics of standing balance between patients with supratentorial stroke and those with infratentorial stroke. This may be attributed to the fact that only one patient with infratentorial stroke had clinically significant ataxia in our group.
There was no significant difference in the WBA between fallers and non-fallers, which is similar to the results of a previous study, which demonstrated no significant correlation between the WBA and the frequency of fall events [24]. However, there was a significant difference in the PSV between fallers and non-fallers. As the difference was more significant in the condition of soft surface than on firm surface, compensation using somatosensory information is assumed to contribute more for preventing falls than compensation using visual information. In particular, the PSVECSS was selected as the only significant predictor for post-stroke falls, except for age, by the multiple logistic regression analysis. This result implies that both the visual and proprioceptive compensation are important for preventing post-stroke falls.
There are several limitations of this study. The amount and intensity of the rehabilitation therapy that subjects received after the BM evaluation was not controlled. However, as the balance function tends to change little after post-stroke 3 months, the rehabilitation therapy was expected to have a limited effect on the balance function. This study included a telephone interview, which is often liable to the recall bias. To minimize the recall bias, subjects were asked fall events that had occurred only within the recent one month prior to the interview. Also, it is hard to say that the subjects represent the stroke population, since the subject could stand without any gait aid or manual contact for at least 30 seconds, and such subjects are usually classified as a mild stroke group. The relatively small number of data could have impeded our search for predictors of post-stroke falls.
In conclusion, we demonstrated the characteristics of the postural instability in patients with stroke, and suggested that advanced age and impaired postural control increase the risk of post-stroke falls. Rehabilitation therapy are suggested to focus on reducing the postural sway with visual and proprioceptive inputs deprived to prevent post-stroke falls.
ACKNOWLEDGMENTS
This study was partially supported by the grant of National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2010-0004974). We thank Dr. Sohee Oh for her statistical advice.
References
1. Herman B, Leyten AC, van Luijk JH, Frenken CW, Op de Coul AA, Schulte BP. Epidemiology of stroke in Tilburg, the Netherlands. The population-based stroke incidence register. 2: Incidence, initial clinical picture and medical care, and three-week case fatality. Stroke. 1982; 13:629–634. PMID: 7123595.

2. Hochstenbach J, Donders R, Mulder T, Van Limbeek J, Schoonderwaldt H. Long-term outcome after stroke: a disability-orientated approach. Int J Rehabil Res. 1996; 19:189–200. PMID: 8910122.
3. Bohannon RW, Leary KM. Standing balance and function over the course of acute rehabilitation. Arch Phys Med Rehabil. 1995; 76:994–996. PMID: 7487452.

4. Fong KN, Chan CC, Au DK. Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Inj. 2001; 15:443–453. PMID: 11350658.

5. Keenan MA, Perry J, Jordan C. Factors affecting balance and ambulation following stroke. Clin Orthop Relat Res. 1984; (182):165–171.

6. Sandin KJ, Smith BS. The measure of balance in sitting in stroke rehabilitation prognosis. Stroke. 1990; 21:82–86. PMID: 2300995.

7. Cathie AG. The influence of the lower extremities upon the structural integrity of the body. J Am Osteopath Assoc. 1950; 49:443–446. PMID: 15415324.
8. Nashner LM, McCollum G. The organization of human postural movements: a formal basis and experimental synthesis. Behav Brain Sci. 1985; 8:135–150.

9. Kamphuis JF, de Kam D, Geurts AC, Weerdesteyn V. Is weight-bearing asymmetry associated with postural instability after stroke? A systematic review. Stroke Res Treat. 2013; 2013:692137. PMID: 23738232.

10. Mackintosh SF, Hill KD, Dodd KJ, Goldie PA, Culham EG. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006; 87:1583–1589. PMID: 17141637.

11. Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008; 45:1195–1213. PMID: 19235120.
12. Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995; 311:83–86. PMID: 7613406.
13. Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995; 26:838–842. PMID: 7740577.
14. Yates JS, Lai SM, Duncan PW, Studenski S. Falls in community-dwelling stroke survivors: an accumulated impairments model. J Rehabil Res Dev. 2002; 39:385–394. PMID: 12173758.
15. Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008; 88:559–566. PMID: 18292215.

16. Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Aust J Physiother. 2001; 47:29–38. PMID: 11552860.

17. Mansfield A, Danells CJ, Zettel JL, Black SE, McIlroy WE. Determinants and consequences for standing balance of spontaneous weight-bearing on the paretic side among individuals with chronic stroke. Gait Posture. 2013; 38:428–432. PMID: 23357758.

18. Chen HY, Wing AM. Independent control of force and timing symmetry in dynamic standing balance: implications for rehabilitation of hemiparetic stroke patients. Hum Mov Sci. 2012; 31:1660–1669. PMID: 22939846.

19. Oken O, Yavuzer G. Spatio-temporal and kinematic asymmetry ratio in subgroups of patients with stroke. Eur J Phys Rehabil Med. 2008; 44:127–132. PMID: 18418332.
20. Garland SJ, Ivanova TD, Mochizuki G. Recovery of standing balance and health-related quality of life after mild or moderately severe stroke. Arch Phys Med Rehabil. 2007; 88:218–227. PMID: 17270520.

21. Marigold DS, Eng JJ. The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture. 2006; 23:249–255. PMID: 16399522.

22. Peurala SH, Kononen P, Pitkanen K, Sivenius J, Tarkka IM. Postural instability in patients with chronic stroke. Restor Neurol Neurosci. 2007; 25:101–108. PMID: 17726268.
23. Pereira LC, Botelho AC, Martins EF. Relationships between body symmetry during weight-bearing and functional reach among chronic hemiparetic patients. Rev Bras Fisioter. 2010; 14:229–266.
24. Sackley CM. Falls, sway, and symmetry of weight-bearing after stroke. Int Disabil Stud. 1991; 13:1–4. PMID: 1917796.

25. Bronstein AM, Buckwell D. Automatic control of postural sway by visual motion parallax. Exp Brain Res. 1997; 113:243–248. PMID: 9063710.

26. Marigold DS, Eng JJ, Tokuno CD, Donnelly CA. Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil Neural Repair. 2004; 18:222–229. PMID: 15537993.

27. Geurts AC, Nienhuis B, Mulder TW. Intrasubject variability of selected force-platform parameters in the quantification of postural control. Arch Phys Med Rehabil. 1993; 74:1144–1150. PMID: 8239951.
28. Lafond D, Corriveau H, Hebert R, Prince F. Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch Phys Med Rehabil. 2004; 85:896–901. PMID: 15179642.
29. de Haart M, Geurts AC, Huidekoper SC, Fasotti L, van Limbeek J. Recovery of standing balance in postacute stroke patients: a rehabilitation cohort study. Arch Phys Med Rehabil. 2004; 85:886–895. PMID: 15179641.
30. Mizrahi J, Solzi P, Ring H, Nisell R. Postural stability in stroke patients: vectorial expression of asymmetry, sway activity and relative sequence of reactive forces. Med Biol Eng Comput. 1989; 27:181–190. PMID: 2601436.

31. Barra J, Oujamaa L, Chauvineau V, Rougier P, Perennou D. Asymmetric standing posture after stroke is related to a biased egocentric coordinate system. Neurology. 2009; 72:1582–1587. PMID: 19414725.

32. Chien CW, Hu MH, Tang PF, Sheu CF, Hsieh CL. A comparison of psychometric properties of the smart balance master system and the postural assessment scale for stroke in people who have had mild stroke. Arch Phys Med Rehabil. 2007; 88:374–380. PMID: 17321832.

33. Liston RA, Brouwer BJ. Reliability and validity of measures obtained from stroke patients using the Balance Master. Arch Phys Med Rehabil. 1996; 77:425–430. PMID: 8629916.

34. Di Fabio RP, Badke MB. Relationship of sensory organization to balance function in patients with hemiplegia. Phys Ther. 1990; 70:542–548. PMID: 2392483.

35. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. PMID: 21414208.

36. Ashburn A, Hyndman D, Pickering R, Yardley L, Harris S. Predicting people with stroke at risk of falls. Age Ageing. 2008; 37:270–276. PMID: 18456791.

37. Baetens T, De Kegel A, Calders P, Vanderstraeten G, Cambier D. Prediction of falling among stroke patients in rehabilitation. J Rehabil Med. 2011; 43:876–883. PMID: 21947179.

38. Mansfield A, Mochizuki G, Inness EL, McIlroy WE. Clinical correlates of between-limb synchronization of standing balance control and falls during inpatient stroke rehabilitation. Neurorehabil Neural Repair. 2012; 26:627–635. PMID: 22275158.

39. Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994; 49:M72–M84. PMID: 8126355.

Fig. 1
The receiver operating characteristics curve of postural sway velocity for post-stroke falls, under condition of eyes closed on soft surface. The cut-off value was 5.30°/s, with a sensitivity of 70.0% and a specificity of 85.3%. The area under the curve (AUC) was 0.822.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download