INTRODUCTION
In a majority of neuromuscular diseases, muscle weakness is observed not only in the muscles of the limbs, but also in the respiratory muscles. Respiratory muscle weakness eventually causes pulmonary function impairment as the disease progresses, which leads to a reduction in the capacity to perform daily activities. Based on the study by Phillips et al. [
1], once forced vital capacity decreases to less than 1 L/min, the probability of death occurring within 1–2 years is high. Therefore, detailed observation and regular assessment of respiratory symptoms are important aspects of patient care [
2]. Precise assessment of pulmonary function and monitoring the progress are essential factors in the continuation of respiratory rehabilitation, which can prevent complications and lower mortality in patients [
34]. To achieve this purpose, standard data for scales that reflect respiratory dynamics need to be used for the assessment of early changes.
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) are known to reflect the weakness of respiratory muscles more sensitively than forced vital capacity measured with a spirometer [
5], and they are widely used to measure the strength of the respiratory muscles. As a result, these three parameters are examined together with general pulmonary function tests in patients with myopathy, with respiratory muscle weakness being the main cause of respiratory failure. However, precise MIP and MEP measurements are less well studied compared with pulmonary function measures, such as tidal volume, forced vital capacity, and forced expiratory volume in 1 second (FEV1) [
6]. Maximal static pressure can detect respiratory muscle weakness more sensitively; therefore, it can become a clinical key parameter for the treatment of respiratory problems in patients with neuromuscular disorders. Therefore, we aimed to measure the standard values for MIP and MEP in healthy Korean children.
Go to :

RESULTS
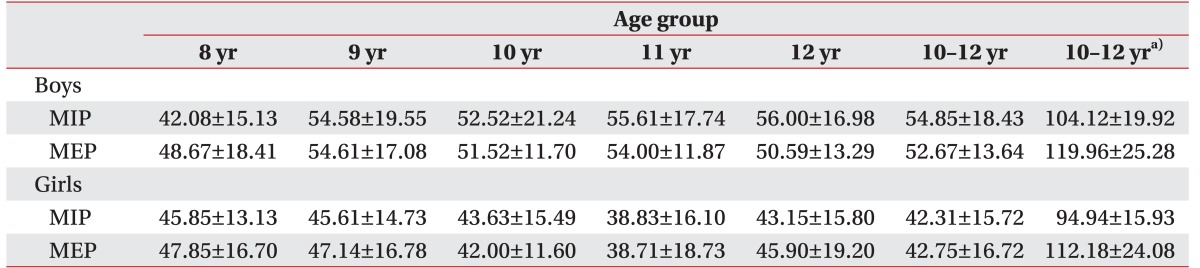
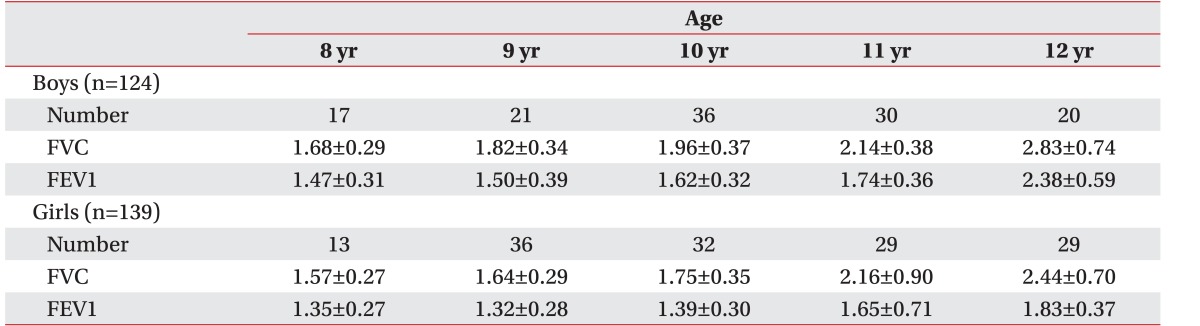
We collected data from 263 subjects. The values (mean±standard deviation) for MIP and MEP in all subjects were 48.46±18.1 cmH
2O and 47.95±16 cmH
2O, respectively. The mean MIP and MEP values for the subjects according to age are shown in
Table 2.
Table 2
MIP and MEP in healthy Korean children

We used variable selection methods (stepwise, forward, and backward methods) to obtain the best correlation among independent values. Statistical values showed strong correlations with respiratory parameters only with use of the backward selection method. MIP was found to have a strong correlation with height (t=-2.471, p=0.014), weight (t=2.189, p=0.030) and mean FEV1 (t=4.323, p=0.000) in boys (Adjusted R
2=0.139). MIP in girls showed a strong correlation with weight (t=2.009, p=0.046; Adjusted R
2=0.011) (
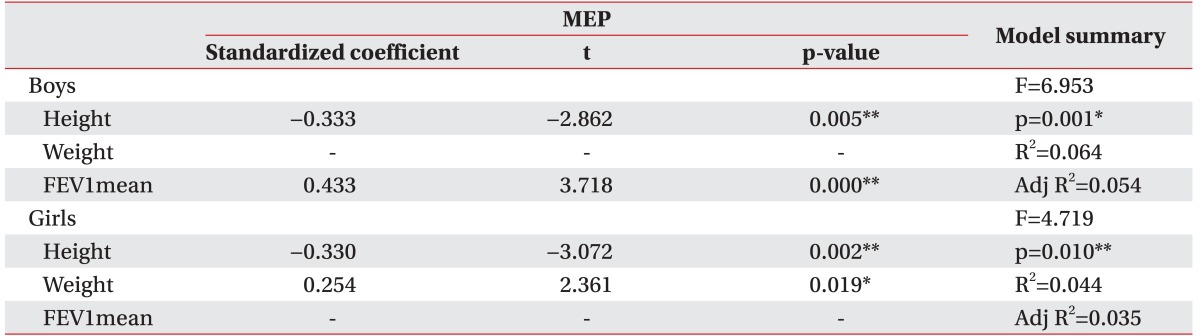
Table 3). MEP in boys also had strong correlations with FEV1 (t=3.718, p=0.000) and height (t=-2.862, p=0.005; Adjusted R
2=0.054), while MEP in girls had strong correlations with weight (t=2.361, p=0.019) and height (t=-3.072, p=0.002; Adjusted R
2=0.035) (
Table 4). Maximal respiratory static pressure, MIP, and MEP values were generally correlated with height. Hence, we assessed whether there was a linear correlation between maximal static pressure and height (
Fig. 1).
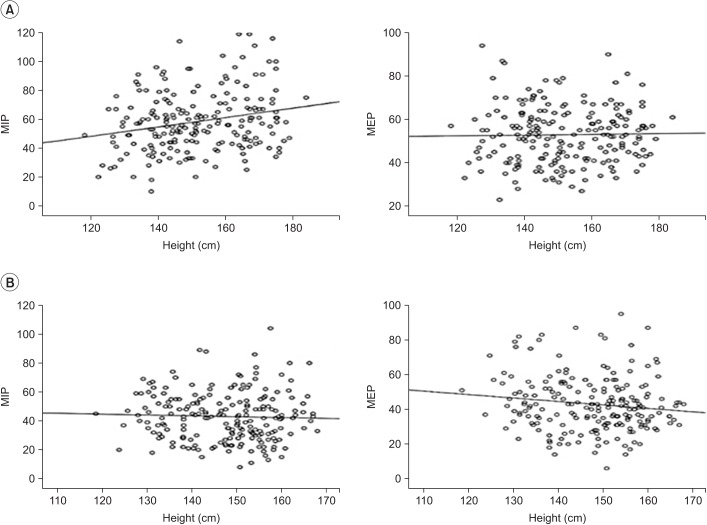 | Fig. 1Linear correlation between maximal static pressures (MIP and MEP) and height in boys (A) and girls (B). MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
|
Table 3
Backward selection method results for MIP
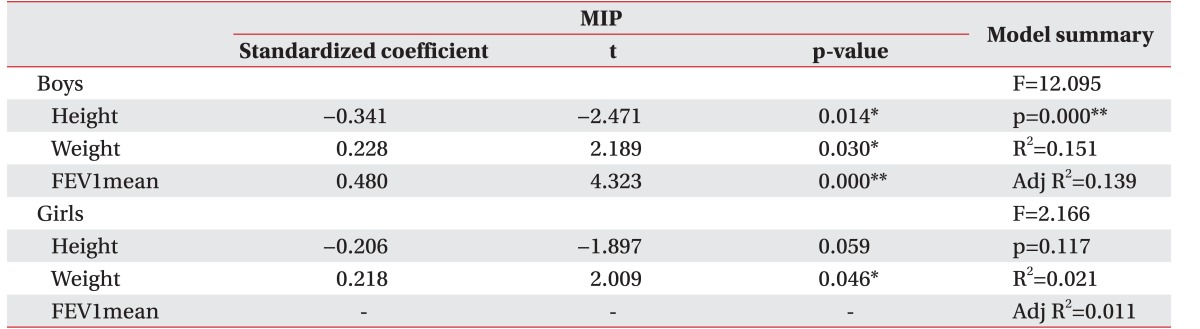

Table 4
Backward selection method results for MEP

Go to :

DISCUSSION
Pulmonary function impairment is an important factor which is closely related to the direct causes of death in majority of neuromuscular disorders. Consequently, pulmonary function tests and maximal static pressure measurements provide objective and reproducible test results in patients with neuromuscular disorders, and are important for establishing the diagnosis and initiating the treatment [
891011].
However, the majority of studies in a healthy Korean population have investigated the predicted or normal values for parameters to confirm the results of basic pulmonary function tests [
612]. Studies from other countries used predictive equations obtained in their respective populations in accordance with their objectives [
41314]. The American Thoracic Society recommends considering ethnicity, age, sex, height, and physical activity while choosing a prediction equation for forced vital capacity [
15]. In our study, we aimed to determine the standard values for MIP and MEP, which could become the criteria for respiratory rehabilitative treatment in patients with neuromuscular disorders.
Maximal respiratory static pressure, MIP, and MEP values were generally correlated with height and weight [
41314]. In our study, height was also an affecting factor for maximal static pressure. Hence, we assessed whether there was a linear correlation between maximal static pressure and height. However, no correlation was found between maximal static pressure and height in the linear correlation graphs (
Fig. 1). We think that these results were due to the difference in growth among subjects. Further analysis that will include more subjects is needed to evaluate this correlation.
The normal static respiratory pressure found in our study of Korean children is different from that in children from other countries. A comparison of our results with those of other studies indicates that standard values cannot be used worldwide. Studies conducted to determine the normal values for pulmonary function in various countries show that clear differences were found between results obtained in different countries. Based on the study by Heinzmann-Filho et al. [
13], the values for MIP and MEP in healthy 10 to 12-year-old children in Brazil were 104.12±19.92 cmH
2O and 119.96±25.28 cmH
2O, respectively, for boys, and 94.94±15.93 cmH
2O and 112.18±24.08 cmH
2O, respectively, for girls (
Table 4). These values are considerably different from the results of our study. Wilson et al. [
14] reported the prediction equations for maximal static pressures in Caucasian adults and children. Using the values obtained in Korean children from our study in the maximal static pressure equation for Caucasian children, the predicted values for mean MEP and MIP in 10-year-old boys and girls were 74.96 cmH
2O and 90.67 cmH
2O, respectively, and 62.16 cmH
2O and 72.94 cmH
2O, respectively. According to our results, MEP and MIP in boys and girls were 51.52 cmH
2O and 52.52 cmH
2O, respectively, and 42.00 cmH
2O and 43.63 cmH
2O, respectively. These results show a difference of more than 20 cmH
2O compared with the criteria for Caucasians and Koreans.
These results may have been affected by several factors. We think that the explanation given by the examiner or the adherence of the examinee had the greatest influence. In addition, based on the study by Dassios et al. [
16], the maximal static pressure measured in patients with cystic fibrosis and healthy subjects can differ because of the testing device or the test method. However, estimating only the examiner or subject variables is incorrect. A difference may also be observed based on body weight, height, or basic pulmonary function, although we could not clearly confirm it in our study [
8141718]. Compiling standard values from one country, ethnicity, or nationality is impossible. Additionally, when comparing standard values for adults, occasionally, no great difference is observed [
19], while at other times a very clear difference is observed [
20]. Our study demonstrates that standard values for children in Korea are different from those for children from other countries and ethnicities. As shown in a study of Navajo children conducted by Arnall et al. [
4], differences based on the regional environment must not be overlooked.
Our study is confined to students of a single elementary school in Yangsan City and its sample size is not large enough to represent children in Korea. It also lacks an analysis of height and weight of the children, which might have influenced the results for MIP and MEP. In turn, a positive correlation between age and MIP, and MEP has not been identified. Further study should include a larger and diverse sample size, and it should be a multicenter study so as to accurately reproduce the average MIP and MEP values for Korean children.
In conclusion, our results showed that boys generally have greater respiratory muscle strength than girls. We found clear differences between the values measured in healthy Korean children and those measured previously in children from other countries. We speculate that the differences are caused by several factors, including ethnicity, nutrition, and daily activity levels. We believe that the results will be helpful to all patients undergoing pulmonary rehabilitation or other respiratory-related treatment.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download