Abstract
Cerebellar mutism (CM) is a rare neurological condition characterized by lack of speech due to cerebellar lesions. CM is often reported in children. We describe a rare case of CM after spontaneous cerebellar hemorrhage. The patient showed mutism, irritability, decreased spontaneous movements and oropharyngeal apraxia. Diffusion tensor imaging revealed significant volume reduction of medial frontal projection fibers from the corpus callosum. In Tracts Constrained by UnderLying Anatomy (TRACULA) analysis, forceps major and minor and bilateral cingulum-angular bundles were not visualized. Cerebello-frontal pathway reconstructed from the FMRIB Software Library showed continuity of fibers, with decreased number of fibers on qualitative analysis. These results suggest that cerebello-frontal disconnection may be a neuroanatomical mechanism of CM. Damage of brain network between occipital lobe, cingulate and cerebellum caused by hemorrhage may also have role in the mechanism of CM in our case.
Cerebellar mutism (CM) is a rare neurological condition characterized by lack of speech after cerebellar injury [1]. Other reported symptoms include irritability, dysphagia, ataxia, gross and fine motor difficulty. This phenomenon usually has been reported following a posterior fossa surgery with cerebellar tumor and rarely has been reported following a stroke [2]. The underlying mechanism of CM is still unclear. Recently, there have been many efforts to investigate the role of a neural circuitry in various brain disorders by diffusion tensor imaging (DTI). However, the functional disconnection in adult CM after a stroke has not been described. In this study, we report the clinical characteristics, DTI findings and 15-month follow-up functional changes in a rare case of mutism after cerebellar hemorrhage in an adult.
A 20-year-old right-handed female presented symptoms of vomiting and dizziness followed by loss of consciousness. The patient showed stupor mentality when she came into the emergency room and the brain computed tomography (CT) and angiography showed a large amount of cerebellar hemorrhage with 4th ventricular collapse from right cerebellar arteriovenous malformation rupture (Fig. 1). Suboccipital craniotomy and hematoma removal with extraventricular drainage was carried out on the day of the incident. After the operation, her mentality gradually improved to a confused state. Brain CT at 3 weeks post-operatively revealed serial resorption of the hematoma and hydrocephalus.
The patient was transferred to rehabilitation department 2 months after the onset of stroke. She was conscious but akinetic and could not follow verbal commands. She was also slightly ataxic and could not walk independently. She was mute and her cognitive and language abilities could not be determined due to her very limited communication. She only showed a few nodding responses without verbal response when presented with simple naming questions. In addition, she had severe dysphagia at oral phase due to oral apraxia. Therefore with these factors, we clinically diagnosed her mutism as CM caused by cerebellar hemorrhage.
After transfer to the rehabilitation department, comprehensive rehabilitation was begun, which included balance and gait training, cognitive training for attention and oromotor stimulation. After a month of comprehensive rehabilitation therapy, she was still unable to speak spontaneously and was only able to mimic [a, i, u] lip posture without phonation. After two months of comprehensive rehabilitation therapy, she was still unable to speak but showed improvements in some understanding and reading abilities. Aphasia quotient (AQ) by Korean version of Western Aphasia Battery was 12.5 points and she was classified as very severe cognitive communication disorder. She showed mild dysmetria with normal muscle strength and started walking with minimal assistance (Berg Balance Scale score 37/56). She was discharged from the hospital and did not receive further rehabilitation therapy.
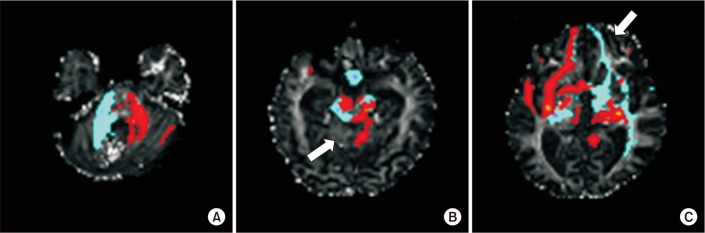
DTI images were obtained six months after the onset of stroke. DTI was acquired using a 3.0-T Philips Achieva MRI scanner (Philips Healthcare, Best, The Netherlands). Images were obtained based on the single-shot spin echo-planar image sequence. The DTI data parameters were: field of view=224 mm×224 mm; matrix=112×109; repetition time (TR)=7,825.63 ms; echo time (TE)=75 ms; slice thickness=2 mm; and b=1,000 s/mm2. We performed imaging analysis using DTIStudio V3.0.2 and Tracts Constrained by UnderLying Anatomy (TRACULA), which are tools used for reconstruction of a set of major white-matter pathways from DTI images. We used the FMRIB Software Library (FSL) for reconstruction of the fronto-cerebellar pathway. Prefrontal cortex was used as the waypoint mask and the entire cerebellum was used as the seed mask. Head motion correction was accomplished by rigid body transformation (rotation and translation, six parameters). Minimum fractional anisotropy (FA) of 0.15 and tract turning-angle of 70° were the parameters for the tract reconstruction with DTIStudio V3.0.2. We analyzed corticospinal tract (CST), arcuate fasciculus (AF), corpus callosum, and inferior longitudinal fasciculus. Two regions of interest (ROIs) were selected for reconstruction of tracts (CST, pontine fibers at the middle pons level and cerebral peduncle at the ventral part of midbrain; AF, anterior part of the superior longitudinal fasciculus and descending portion of the SLF in the posterior temporal lobe). Whole corpus callosum in a midsagittal view was used as a ROI for corpus callosum reconstruction. Significant loss of corpus callosum fibers projecting on bilateral medial frontal cortex was evident, with no abnormalities in the AF, CSTs and inferior longitudinal fasciculus (Fig. 2). In a single healthy age-matched control, the reconstructed corpus callosum fibers revealed bilateral extensions on both hemispheres. TRACULA reconstructed 18 major white-matter tracts. In our case, forceps major, forceps minor and bilateral cingulum-angular bundles were not visualized compared to one normal age-matched control (Fig. 3). Fronto-cerebellar pathway reconstructed by FSL showed some decreased fronto-cerebellar fibers between the right cerebellum and left frontal cortex with continuity of fibers (Fig. 4).
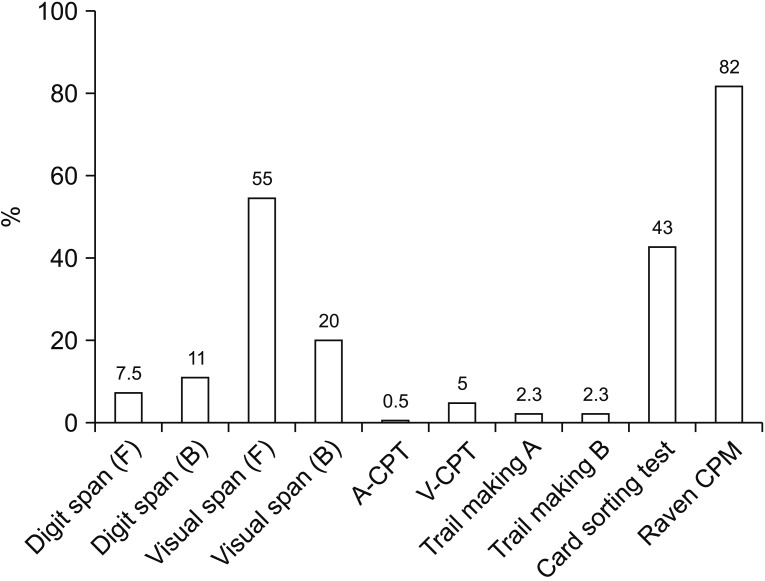
Fifteen months after the stroke, the patient was able to ambulate independently, grasp and release freely with normal strength in all limbs. Her mutism improved and although she started phonation, severe dysarthria still persisted, which resulted in reading difficulty and the adoption of a letter-by-letter reading strategy. The Korean version of Mini-Mental State Examination displayed an improved score of 27/30. The computer-based cognitive evaluation consisting of digit span, visual span, continuous performance task, trail making, card sorting test and Raven's Coloured Progressive Matrices test revealed impaired attention and executive function (Fig. 5).
Our case presented rare symptoms including severe CM and akinesia after cerebellar hemorrhagic stroke. Tractography using DTI suggested that cerebello-frontal disconnection, especially in medial frontal lobe, may be a neuroanatomical mechanism of CM in this case.
CM is reported commonly after resection of cerebellar tumors in children. A prospective study recruiting 148 pediatric patients with resection of cerebellar tumor revealed that 27.7% of these patients were cases of CM [2]. In comparison, only 1.2% of adult posterior fossa surgery displayed CM syndrome indicating lower frequency in adults than children [3]. The incomplete development of motor speech control and language has been suggested as the reason of frequent incidence of CM in children [2]. Akinetic mutism after cerebellar stroke has rarely been reported, although the frontal lobe, basal ganglia, mesencephalon and thalamus have been reported as possible lesions [4]. Secondary reactions after the posterior fossa tumor resection result in edema, perfusional disturbances, disturbances in neurotransmitter release and axonal injury, which may result in a differing incidence between cerebellar stroke and tumor [3].
The pathomechanism of CM has not been well understood yet. Some investigators propose that mutism itself is mediated primarily by crossed cerebello-cerebral diaschisis and chronic symptoms after mutism is mediated by direct cerebellar injury [1]. Recent neuroimaging studies support the damage of cerebello-thalamo-cerebral (CTC) pathway as an underlying mechanism of CM. Quantified Tc-99m-ECD SPECT studies showed perfusional deficits in the bilateral prefrontal brain areas in a right cerebellar hemorrhage adult patient with CM [5]. In an examination of pediatric patients following posterior fossa tumor surgery, the CM group showed significant diminished volumes in fronto-cerebellar association fibers in tractography [6]. Also, a recent DTI study reported that the CTC pathway connecting the right cerebellum with left frontal regions (areas for speech production and expressive language) may be disrupted in CM [7]. In our case, DTI tractography revealed diminished fiber tracts in bilateral medial frontal connections via corpus callosum and the cingulum. Also decreased corpus callosum fibers and absence of forceps major and minor were noted in our case. Decreased number fibers and changes of FA and diffusivities on DTI study may due to the pressure effects of white matter tracts in hydrocephalus [8]. However, a DTI study with voxel-by-voxel basis showed positive correlation of FA value in bilateral cerebellar peduncles, right occipital lobe, and right anterior cingulate with specific tasks [9]. Severe attention deficit in this case may be related to damage of the brain network between the occipital lobe, cingulate and after cerebellum due to hemorrhage. The patient also showed difficulty in reading (dyslexia) with the need of a letter-by-letter reading strategy even after the mutism disappeared. Decreased white matter tracts in corpus callosum in DTI and absence of forceps major in TRACULA may also explain the alexia as the result of disruption interhemispheric visual tract in this case. Cerebello-frontal pathway reconstructed by FSL showed fibers continuity with decreased fibers numbers on qualitative analysis. Decreased cerebello-frontal fibers tract and FA value in cerebellar mutism have been described, although quantitative analysis was not available in the patient. These findings support the hypothesis that fronto-cerebellar disconnection is the primary mechanism in CM. The similarity of pathophysiology between akinetic mutism and autism spectrum disorder was recently reported [10]. Neuroimaging studies in autism spectrum disorders showed underconnectivity via the corpus callosum, and one of five children with behavioral changes met the criteria of autism disorder according to the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition) in pediatric patients after posterior fossa tumor removal [10], which includes impairments in social interactions, communication, behavior, interests and activities.
Another clinical significance of our case is the long-term follow-up duration of the behavioral symptoms. She needed fifteen months until the clearance from mutism followed by severe dysarthria. Her mutistic phase was much longer than the generally reported duration of CM, which usually lasts a few weeks to several months [2]. Postmutistic dysarthria, language disorder and behavioral disturbance have been reported in CM [1]. Our case also had severe dysarthria, attention deficit and executive dysfunction even after resolution of CM.
In conclusion, we describe a case of rare CM in an adult patient with cerebellar stroke. The tractography results suggest that cerebello-frontal disconnection may be a neuroanatomical mechanism of CM, and damage of brain network between occipital lobe, cingulate and cerebellum caused by hemorrhage may also have role in the mechanism of CM in our case.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIP) (No. 2016R1A2B4009206).
References
1. Kuper M, Timmann D. Cerebellar mutism. Brain Lang. 2013; 127:327–333. PMID: 23398780.
2. Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the Posterior Fossa Syndrome in children after cerebellar tumour surgery. Cortex. 2010; 46:933–946. PMID: 20116053.

3. Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D, et al. Complications of posterior cranial fossa surgery: an institutional experience of 500 patients. Surg Neurol. 2009; 72:369–375. PMID: 19604553.
4. Nagaratnam N, Nagaratnam K, Ng K, Diu P. Akinetic mutism following stroke. J Clin Neurosci. 2004; 11:25–30. PMID: 14642361.

5. Marien P, Verslegers L, Moens M, Dua G, Herregods P, Verhoeven J. Posterior fossa syndrome after cerebellar stroke. Cerebellum. 2013; 12:686–691. PMID: 23575947.

6. Soelva V, Hernaiz Driever P, Abbushi A, Rueckriegel S, Bruhn H, Eisner W, et al. Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Childs Nerv Syst. 2013; 29:597–607. PMID: 23184224.

7. Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Strother D, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol. 2012; 14:1294–1303. PMID: 22952198.

8. Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol. 2006; 27:1717–1724. PMID: 16971621.
9. Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci Lett. 2010; 477:72–76. PMID: 20416360.

10. Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000; 123(Pt 5):1051–1061. PMID: 10775549.

Fig. 1
Computed tomography scan showing a large amount of cerebellar hemorrhage (A) and tangled vascular structure (arrowhead) (B).
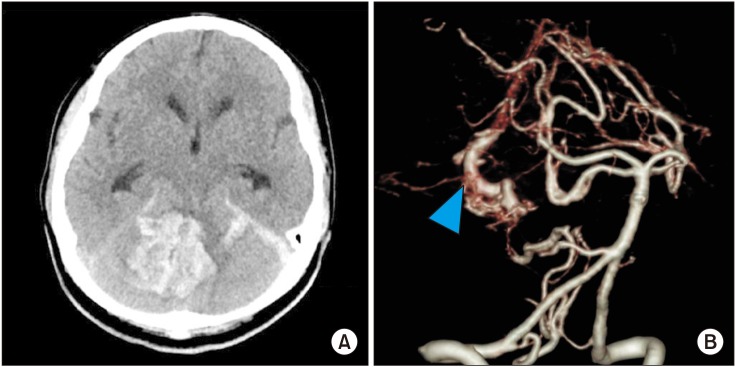
Fig. 2
Corticospinal tract (A), arcuate fasciculus (B), axial view (C) and sagittal view (D) of the corpus callosum with significant fiber loss projecting on medial frontal cortex (arrow).

Fig. 3
All tracts reconstructed by TRACULA (Tracts Constrained by UnderLying Anatomy). Forceps major, forceps minor (arrowhead) (A-a), and bilateral cingulum-angular bundles (arrow) (A-b) of a healthy control were not visualized in our case (B-a, B-b).

Fig. 4
Fronto-cerebellar pathway reconstructed by the FMRIB Software Library (FSL). (A) At the cerebellar hemorrhage level. (B) Decreased fronto-cerebellar fibers (arrows) between the right cerebellum and left frontal cortex was seen at the caudal midbrain level of cerebellar decussation and (C) prefrontal area.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download