1. Ahmad S, Muzamil A, Lateef M. A Study of incidence and etiopathology of vocal cord paralysis. Indian J Otolaryngol Head Neck Surg. 2002; 54:294–296. PMID:
23119914.

2. Syamal MN, Benninger MS. Vocal fold paresis: a review of clinical presentation, differential diagnosis, and prognostic indicators. Curr Opin Otolaryngol Head Neck Surg. 2016; 24:197–202. PMID:
27092906.
3. Xu W, Han D, Hou L, Zhang L, Zhao G. Value of laryngeal electromyography in diagnosis of vocal fold immobility. Ann Otol Rhinol Laryngol. 2007; 116:576–581. PMID:
17847724.

4. Ingle JW, Young VN, Smith LJ, Munin MC, Rosen CA. Prospective evaluation of the clinical utility of laryngeal electromyography. Laryngoscope. 2014; 124:2745–2749. PMID:
24715712.

5. Weddell G, Feinstein B, Pattle E. The electrical activity of voluntary muscle in man under normal and pathological conditions. Brain. 1944; 67:178–257.

6. Faaborg-Andersen K, Buchthal F. Action potentials from internal laryngeal muscles during phonation. Nature. 1956; 177:340–341. PMID:
13297038.

7. Faaborg-Andersen K, Edfeldt AW. Electromyography of intrinsic and extrinsic laryngeal muscles during silent speech: correlation with reading activity. Acta Otolaryngol. 1958; 49:478–482. PMID:
13605726.
8. Buchthal F. Electromyography of intrinsic laryngeal muscles. Q J Exp Physiol Cogn Med Sci. 1959; 44:137–148. PMID:
13645900.

9. Kotby MN. Percutaneous laryngeal electromyography. Standardization of the technique. Folia Phoniatr (Basel). 1975; 27:116–127. PMID:
1193500.
10. Guha K, Sabarigirish K, Singh SK, Yadav A. Role of laryngeal electromyography in predicting recovery after vocal fold paralysis. Indian J Otolaryngol Head Neck Surg. 2014; 66:394–397. PMID:
26396950.

11. Smith LJ, Rosen CA, Munin MC. Vocal fold motion outcome based on excellent prognosis with laryngeal electromyography. Laryngoscope. 2016; 126:2310–2314. PMID:
27242070.

12. Pardo-Maza A, Garcia-Lopez I, Santiago-Perez S, Gavilan J. Laryngeal electromyography for prognosis of vocal fold paralysis. J Voice. 2017; 31:90–93. PMID:
27068040.

13. Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010; 17(Suppl 1):28–30. PMID:
20590805.

14. Wang CC, Chang MH, Jiang RS, Lai HC, De Virgilio A, Wang CP, et al. Laryngeal electromyography-guided hyaluronic acid vocal fold injection for unilateral vocal fold paralysis: a prospective long-term follow-up outcome report. JAMA Otolaryngol Head Neck Surg. 2015; 141:264–271. PMID:
25590517.
15. Bachor E, Bonkowsky V, Hacki T. Herpes simplex virus type I reactivation as a cause of a unilateral temporary paralysis of the vagus nerve. Eur Arch Otorhinolaryngol. 1996; 253:297–300. PMID:
8737789.

16. Parano E, Pavone L, Musumeci S, Giambusso F, Trifiletti RR. Acute palsy of the recurrent laryngeal nerve complicating Epstein-Barr virus infection. Neuropediatrics. 1996; 27:164–166. PMID:
8837078.

17. Parnes SM, Satya-Murti S. Predictive value of laryngeal electromyography in patients with vocal cord paralysis of neurogenic origin. Laryngoscope. 1985; 95:1323–1326. PMID:
4058209.

18. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11:93–98. PMID:
8721066.

19. Perotto AO. Anatomical guide for the electromyographer: the limbs and trunk. 5th ed. Springfield: Charles C Thomas Publisher;2011. p. 311–316.
20. Kimaid PA, Crespo AN, Moreira AL, Wolf AE, Franca-Jr MC. Laryngeal electromyography techniques and clinical use. J Clin Neurophysiol. 2015; 32:274–283. PMID:
26241236.

21. Garcia-Lopez I, Santiago-Perez S, Penarrocha-Teres J, del Palacio AJ, Gavilan J. Laryngeal electromyography in diagnosis and treatment of voice disorders. Acta Otorrinolaringol Esp. 2012; 63:458–464. PMID:
22770868.
22. Sulica L, Blitzer A. Electromyography and the immobile vocal fold. Otolaryngol Clin North Am. 2004; 37:59–74. PMID:
15062687.

23. Booka E, Takeuchi H, Nishi T, Matsuda S, Kaburagi T, Fukuda K, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore). 2015; 94:e1369. PMID:
26287423.

24. Sato Y, Kosugi S, Aizawa N, Ishikawa T, Kano Y, Ichikawa H, et al. Risk factors and clinical outcomes of recurrent laryngeal nerve paralysis after esophagectomy for thoracic esophageal carcinoma. World J Surg. 2016; 40:129–136. PMID:
26464155.

25. Kurihara N, Imai K, Minamiya Y, Saito H, Takashima S, Kudo S, et al. Hoarseness caused by arytenoid dislocation after surgery for lung cancer. Gen Thorac Cardiovasc Surg. 2014; 62:730–733. PMID:
23807399.

26. Calo PG, Medas F, Erdas E, Pittau MR, Demontis R, Pisano G, et al. Role of intraoperative neuromonitoring of recurrent laryngeal nerves in the outcomes of surgery for thyroid cancer. Int J Surg. 2014; 12(Suppl 1):S213–S217. PMID:
24862668.
27. Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:439–443. PMID:
25136863.
28. De Virgilio A, Chang MH, Jiang RS, Wang CP, Wu SH, Liu SA, et al. Influence of superior laryngeal nerve injury on glottal configuration/function of thyroidectomy-induced unilateral vocal fold paralysis. Otolaryngol Head Neck Surg. 2014; 151:996–1002. PMID:
25214548.

29. Wang CC, Chang MH, De Virgilio A, Jiang RS, Lai HC, Wang CP, et al. Laryngeal electromyography and prognosis of unilateral vocal fold paralysis: a long-term prospective study. Laryngoscope. 2015; 125:898–903. PMID:
25346497.
30. Crumley RL. Unilateral recurrent laryngeal nerve paralysis. J Voice. 1994; 8:79–83. PMID:
8167791.

31. Paquette CM, Manos DC, Psooy BJ. Unilateral vocal cord paralysis: a review of CT findings, mediastinal causes, and the course of the recurrent laryngeal nerves. Radiographics. 2012; 32:721–740. PMID:
22582356.

32. Leder SB, Suiter DM, Duffey D, Judson BL. Vocal fold immobility and aspiration status: a direct replication study. Dysphagia. 2012; 27:265–270. PMID:
21858715.

33. Bou-Malhab F, Hans S, Perie S, Laccourreye O, Brasnu D. Swallowing disorders in unilateral recurrent laryngeal nerve paralysis. Ann Otolaryngol Chir Cervicofac. 2000; 117:26–33. PMID:
10671711.
34. Taniyama Y, Miyata G, Kamei T, Nakano T, Abe S, Katsura K, et al. Complications following recurrent laryngeal nerve lymph node dissection in oesophageal cancer surgery. Interact Cardiovasc Thorac Surg. 2015; 20:41–46. PMID:
25312996.

35. Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003; 550(Pt 1):287–304. PMID:
12754311.

36. Kimaid PA, Crespo AN, Quagliato EM, Wolf A, Viana MA, Resende LA. Laryngeal electromyography: contribution to vocal fold immobility diagnosis. Electromyogr Clin Neurophysiol. 2004; 44:371–374. PMID:
15473350.
37. Rickert SM, Childs LF, Carey BT, Murry T, Sulica L. Laryngeal electromyography for prognosis of vocal fold palsy: a meta-analysis. Laryngoscope. 2012; 122:158–161. PMID:
22147604.

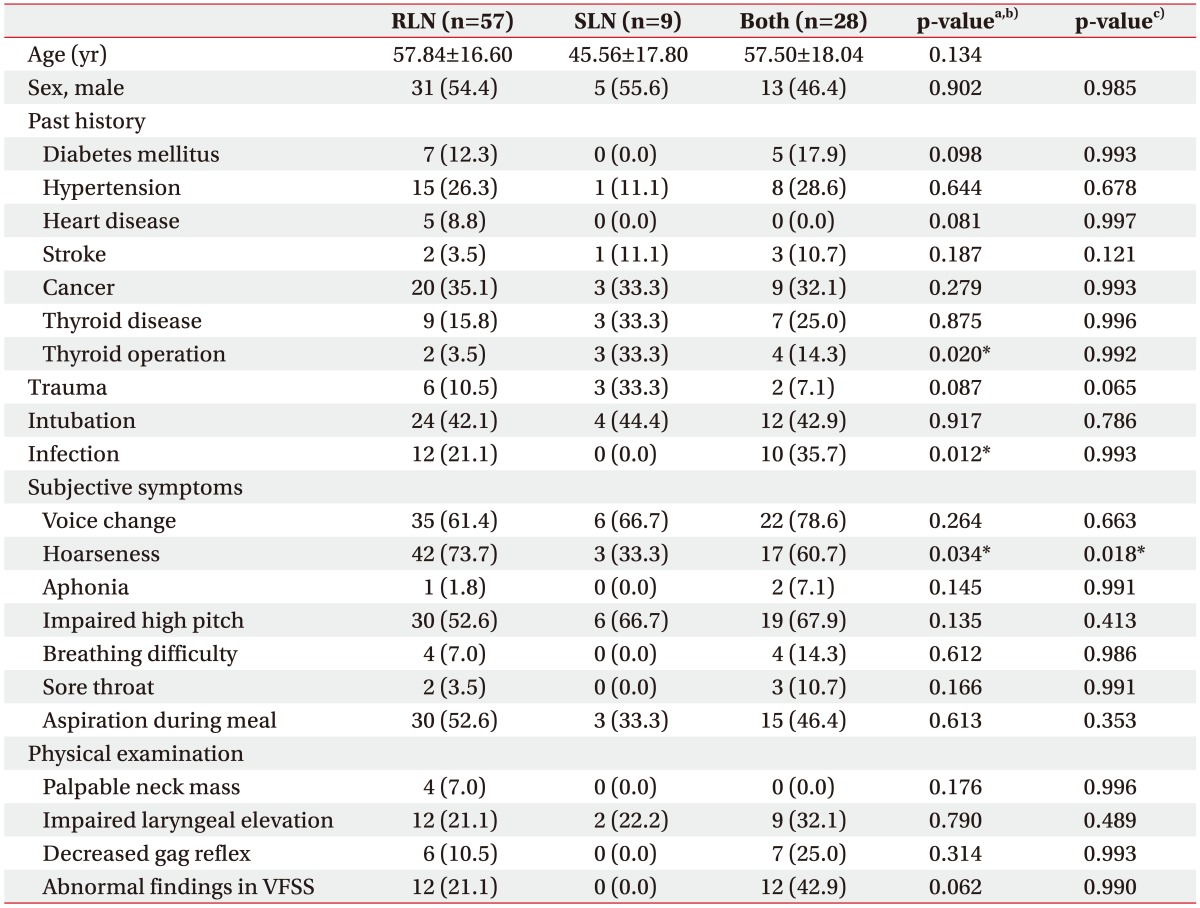
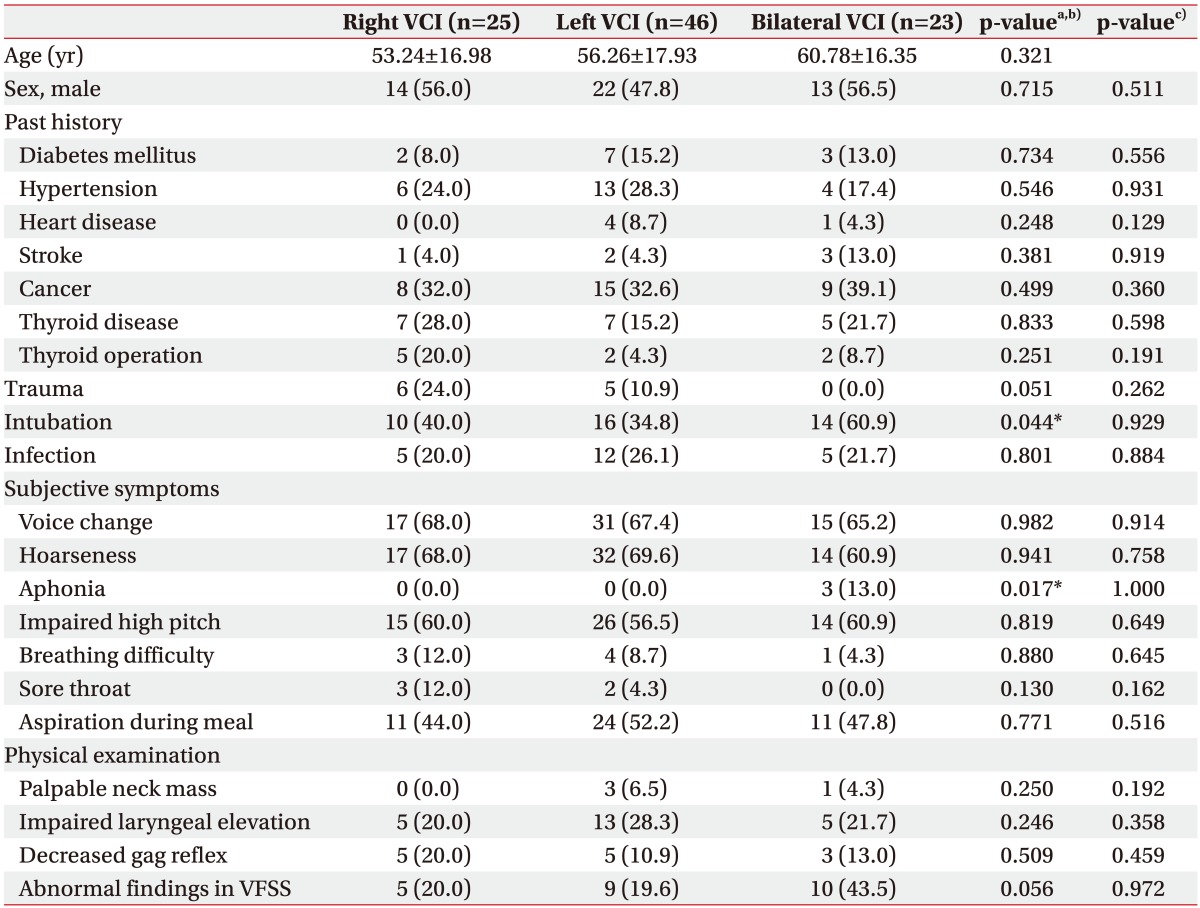




 PDF
PDF ePub
ePub Citation
Citation Print
Print



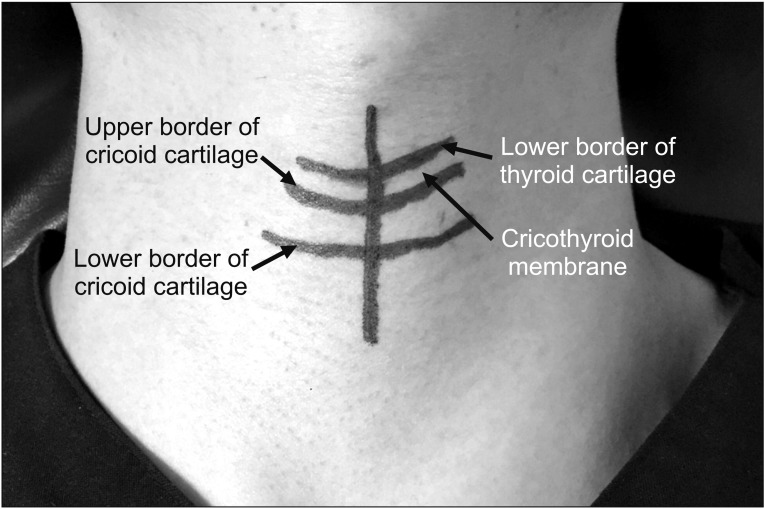
 XML Download
XML Download