Abstract
Bilateral paramedian thalamic infarction is a rare subtype of stroke caused by occlusion of the artery of Percheron, an uncommon variant originating from one of the posterior cerebral arteries. This type of stroke has several major clinical presentations: altered mental status, behavioral amnestic impairment, aphasia or dysarthria, ocular movement disorders, motor deficits, cerebellar signs, and others. Few cases of bilateral paramedian thalamic infarction-related pseudobulbar palsy characterized by dysarthria, dysphagia, and facial and tongue weakness have been reported. We report here a rare case of acute severe pseudobulbar palsy as a manifestation of bilateral paramedian thalamic infarction.
Thalamic infarctions are commonly unilateral; bilateral thalamic infarctions occur infrequently in patients with anomalous thalamus perfusion [1]. Paramedian infarction is the second most common type of thalamic infarction, after thalamogeniculate infarction, and is characterized by several neuropsychologic, oculomotor, and consciousness disturbances. While normally only one paramedian artery arises from a single P1 segment, in some, both paramedian arteries arise from a common P1 trunk. This anatomic variant is known as the artery of Percheron. Occlusion of this artery is rare and results in bilateral paramedian thalamic infarction [2], typically characterized by a triad of altered mental status, vertical gaze palsy, and memory impairment.
A rare case of clinical manifestation of pseudobulbar palsy is reported in [3]. The patient presented with weakness in the muscles regulating swallowing, speech, and chewing because of an anatomical variation, the artery of Percheron, which resulted in bilateral paramedian thalamic infarct.
A 61-year-old male visited the emergency room with sudden-onset dysarthria and dysphagia without definite motor impairment. He was a chronic alcoholic and had been on regular medication for hypertension and diabetes mellitus. He was afebrile, had a regular pulse (79 beats/min) and his blood pressure was 149/80 mmHg.
Laboratory investigations were unremarkable, with the exception of mildly elevated levels of alcohol (10.1 mg/dL; normal range, <10 mg/dL) and fibrinogen (359 mg/dL; normal range, 170–350 mg/dL). The result of a platelet function test was 11 U (normal range, 64–156 U).
Upon neurological examination, the patient was irritable and his cognition was slightly impaired, with a Mini-Mental State Examination (MMSE) score of 25/30. He could not answer questions related to time orientation, recall, calculation, or parts of language correctly. His pupils were isocoric with symmetric pupillary light reflexes. His facial and buccal sensation was normal, but he showed upward gaze limitation in the gaze test (Fig. 1). He was dysarthric and had a hypernasal voice. Swallowing was difficult and he frequently choked on his saliva. The soft palate drooped bilaterally; the gag reflex was present. Tongue movements were slow and of limited range. Tongue protrusion was markedly weak without deviation. A cerebellum function test revealed that his hands were tremorous during the finger-to-nose test, while performance on the heel-to-shin test was intact bilaterally. His sensory function was intact, with the exception of proprioception and touch on the lower limbs.
An emergent enhanced brain computed tomography (CT) revealed the possibility of bilateral thalamic infarction and mild atherosclerotic changes in the left and right cavernous and petrous internal carotid artery (ICA), left ICA origin, and the V4 segment of the left vertebral artery with no significant stenosis, later confirmed by magnetic resonance imaging (MRI) as areas of restricted diffusion. MRI showed symmetrical high signal intensity in both thalami on diffusion-weighted and fast spin-echo T2-weighted images and low signal intensity on apparent diffusion coefficient maps at the same level, while no brainstem lesion was found. CT angiography showed an artery of Percheron (arrowhead) originating from the left P1 posterior cerebral artery (Fig. 2). Thus, we diagnosed a bilateral paramedian thalamic infarction.
Three days after onset, an initial assessment of dysphagia was performed. A videofluoroscopic swallowing study (VFSS) showed severe dysfunction. In the oral phase, motor control including lip sealing, tongue control, and mastication was inadequate and oral transit time was delayed with tongue base residue. In the pharyngeal phase, laryngeal elevation was severely decreased and pharyngeal transit time was prolonged with slurred pharyngeal wall movement; 50% of food material remained in vallecular and pyriform sinuses after swallowing.
The patient was transferred to a rehabilitation unit after acute-stage management in the Department of Neurology. An initial speech assessment performed 9 days after symptom onset revealed severe dysarthria with hypernasality, palilalia, and imprecise consonants. Alternating motion rates (AMRs) and sequential motion rates (SMRs) were reduced and speech intelligibility decreased to 50%–55%. The patient underwent dysphagia treatments such as oromotor exercises, pharyngeal tactile stimulation, tongue retraction exercises, effortful swallowing exercises, vocal cord adduction exercises, the Mendelsohn maneuver, and shaker exercises. He also received respiration-phonation training, and dysarthria treatment.
Motor- and sensory-evoked potential tests were also performed. There was no definite abnormal finding upon motor-evoked potential stimulation, revealing no cortical pathway dysfunction when the bilateral abductor pollicis brevis muscle and abductor halluces muscle were stimulated. For somatosensory evoked potential stimulated on both limbs, both median nerve latencies recorded in N20 were in the normal range. However, when both posterior tibial nerves were stimulated, increased latencies in P1 were observed. The latency was 43.50 ms for the right side and 43.40 ms for the left side (Fig. 3). These results were suggestive of somatosensory dysfunction.
The follow-up VFSS was performed 4 weeks after the first test, 31 days after symptom onset. Oral transit time and motor control improved but bolus formation and propulsion remained inadequate, with tongue base residue. Decreased laryngeal elevation and pharyngeal time and residue of the vallecular and pharyngeal wall were still observed, but no aspiration or penetration occurred during tests with dysphagia diets 2 (Toromi-up A, 2 g per liquid 100 mL; Nisshin OilliO, Tokyo, Japan) and 3 (Toromi-up A, 1 g per liquid 100 mL; Nisshin OilliO)
One month after the infarction, dysarthria had improved to moderate severity; speech intelligibility was 60%–65%. AMRs and SMRs were still reduced. The percentage of correct consonants was 86.05%. The MMSE score improved from 25 to 27. The patient was discharged after 34 days in hospital.
Pseudobulbar palsy symptoms remained at discharge and dysphagia and dysarthria treatment continued for 1 month. He exhibited upward gaze palsy during an outpatient visit at 6 months after discharge.
We present a rare case of acute severe pseudobulbar palsy as a manifestation of bilateral paramedian thalamic infarction. When bilateral medial thalamic infarcts are found, occlusion of the artery of Percheron should be considered as the main diagnosis.
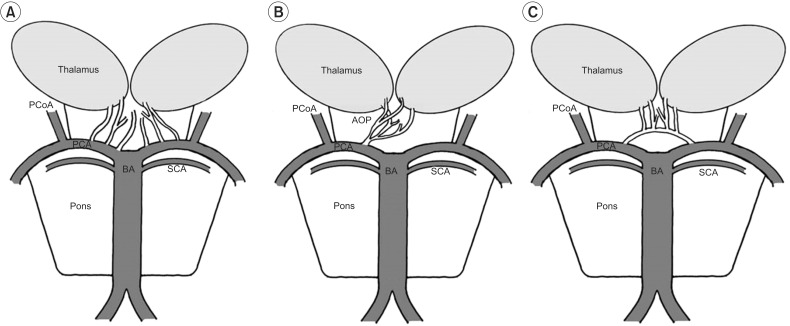
Normally, the thalami and midbrain receive blood supply from both the anterior (internal carotid arteries) and posterior (vertebra-basilar system) circulation. The posterior circulation supplies the medial aspects via branches arising from the P1 segments, and the lateral and superior aspects via branches arising from the P2 segments of the posterior cerebral arteries. Percheron described three possible variations involving the paramedian thalamic-mesencephalic arterial supply (Fig. 4). The second variant, consisting of a common trunk arising from one of the P1 segments providing bilateral distribution, is known as the artery of Percheron [4]. Given that the clinical manifestations are related to the topography of the lesions, we believe that both infarctions were located in the anterolateral or medial thalamic territory. Bilateral involvement of the anteroventral and ventrolateral nuclei was the most likely. Recent studies reveal projections from the anteroventral nucleus to the caudal and medial orbitofrontal cortex. Similarly, the ventrolateral nucleus possesses reciprocal and topically arranged diffuse connections with cortical areas 4 and 6, and the medial parts of this nucleus possess connections with the face cortical areas [5]. Thus, we speculate that bithalamic lesioning of these nuclei completely interrupted the thalamocortical projections to the face, resulting in acute pseudobulbar palsy via diaschisis [6]. This diaschisis phenomenon depends on thalamocortical interconnections and provides corroborating physiological evidence for the hypothesis of distributed neural circuits and a mechanism for the behavioral deficits in patients with focal thalamic lesions.
If the lesion involves corticobulbar fibers but not corticospinal neurons, then there will be no paralysis of the upper or lower extremities, as reported here. Lesions of medial thalamus disrupt the corticofugal fibers that lead from the motor and premotor cortices to the nucleus of Darkschewitsch and the interstitial nucleus of Cajal in the midbrain, which are concerned with vertical gaze (up and down).
Our case presents several important clinical variations. Firstly, altered mental status can present anywhere on the spectrum from drowsiness to coma. Our patient demonstrates that a bilateral paramedian infarct may present with no loss of consciousness, but mild cognitive impairment and irritability. This differs greatly from previous case reports [78] of difficulty maintaining normal consciousness for several days. The decreased level of consciousness is reportedly due to affected midline thalamic nuclei, which extend rostrally from the reticular activating system [8]. Secondly, the patient showed no motor weakness. Dysarthria and dysphagia without other neurologic deficits are indications for screening for lacunar infarction. Also, MRI showed a focal infarction isolated to the bilateral paramedian thalami while the remainder of the hemispheres and the brain stem appeared normal (Fig. 2). These findings suggest that this syndrome is due to an interruption of the supranuclear fibers to the midbrain vergence neurons.
Thirdly, based on the patient's alcohol history and hand tremors, alcohol withdrawal seizure and delirium tremens were considered. Further, we were aware that chronic alcoholism might have affected cognition or resulted in sensory symptoms.
To our knowledge, this is the second report that bilateral thalamic infarction on specific nuclei could manifest as acute pseudobulbar palsy. Physicians should be aware that upward gaze palsy coupled with dysarthria and dysphagia may be a presentation of bilateral thalamic infarction.
References
1. Song YM. Topographic patterns of thalamic infarcts in association with stroke syndromes and aetiologies. J Neurol Neurosurg Psychiatry. 2011; 82:1083–1086. PMID: 21406535.

3. Karacostas D, Artemis N, Giannopoulos S, Milonas I, Bogousslavsky J. Bilateral thalamic infarcts presenting as acute pseudobulbar palsy. Funct Neurol. 1994; 9:265–268. PMID: 7750810.
4. Matheus MG, Castillo M. Imaging of acute bilateral paramedian thalamic and mesencephalic infarcts. AJNR Am J Neuroradiol. 2003; 24:2005–2008. PMID: 14625223.
5. Carpenter MB. The diencephalon. In : Carpenter MB, editor. Human neuroanatomy. 7th ed. Baltimore: Williams & Wilkins;1976. p. 453–454.
7. Teoh HL, Ahmad A, Yeo LL, Hsu E, Chan BP, Sharma VK. Bilateral thalamic infarctions due to occlusion of artery of Percheron. J Neurol Sci. 2010; 293:110–111. PMID: 20417939.

8. Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry. 1987; 50:900–909. PMID: 3625213.

Fig. 1
Upward gaze limitation in nine diagnostic positions of gaze. When asked to look in the 9 diagnostic positions of gaze (up and to the right, up, up and to the left, right, straight ahead, left, down and to the right, down, and down and to the left) the upper gaze was limited (first row).
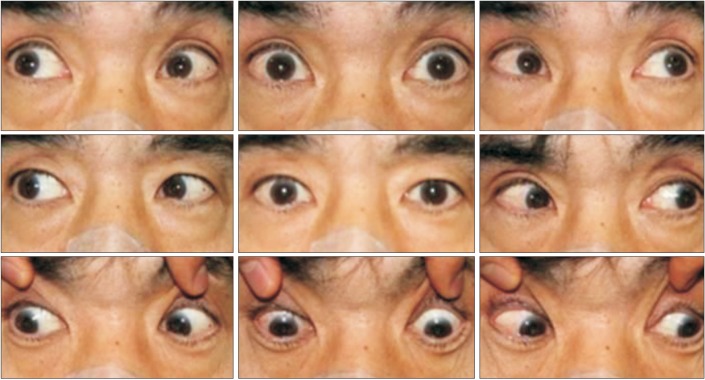
Fig. 2
Initial brain computed tomography (CT) angiography and magnetic resonance images. (A) Axial diffusion-weighted imaging sequences. (B) Apparent diffusion coefficient image. (C) Coronal T2 image showing an acute bilateral paramedian thalamic infarction (arrows). (D) CT angiography. (E) Magnified image of the basilar and posterior cerebral arteries demonstrating an unpaired thalamic perforating artery (arrowhead) arising from the proximal P1 segment of the left posterior cerebral artery supplying the bilateral thalami. (F) Axial T2 FLAIR image showing no midbrain lesion.
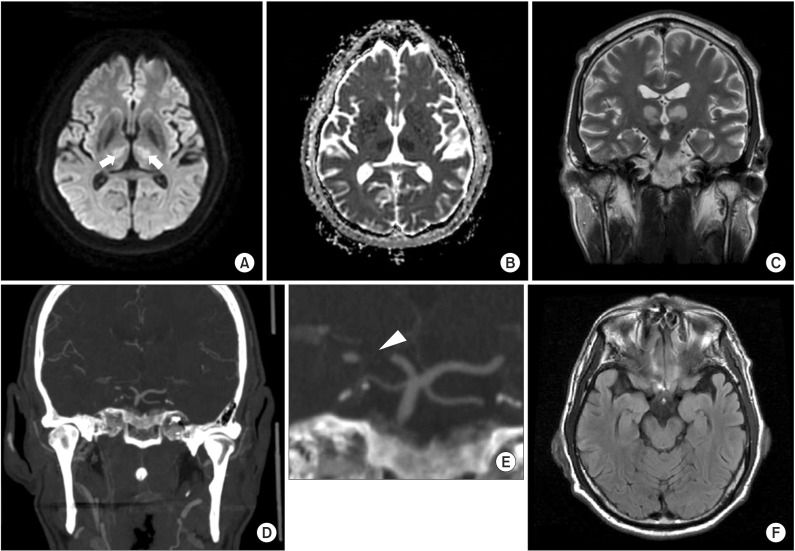
Fig. 3
Increased latencies for both posterior tibial nerves representing somatosensory dysfunction. When upper extremities were stimulated for somatosensory evoked potentials, both median nerves were within the normal range (A, B). However, increased latencies were observed for both posterior tibial nerves when the lower extremities were stimulated for somatosensory evoked potentials (C, D).
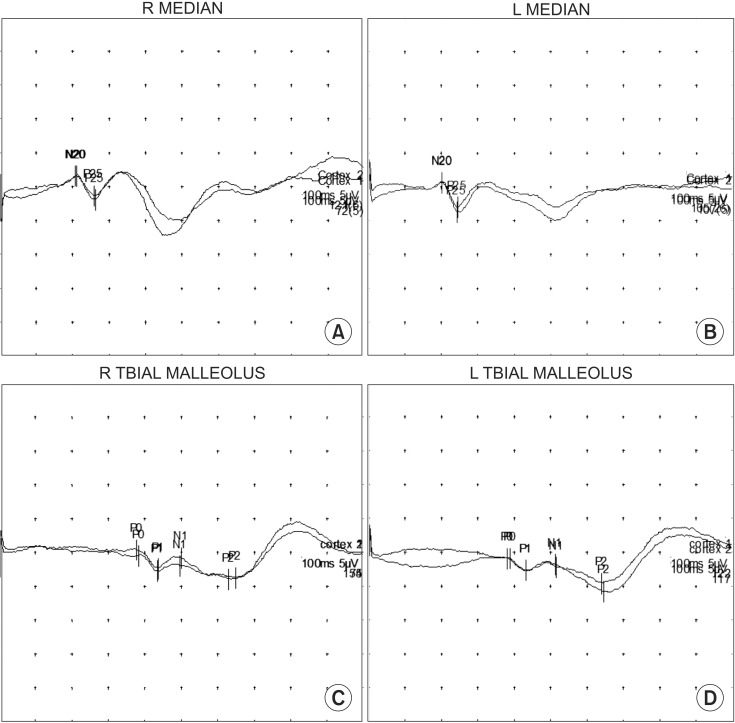
Fig. 4
Schematic representation of variations of the paramedian thalamic-mesencephalic arterial supply according to Percheron. (A) In the most common variation, many small perforating arteries arise from the P1 segment of the PCA. (B) The AOP is a single perforating blood vessel arising from a P1 segment. (C) In the third variant, an arcade of perforating branches arises from an artery bridging the P1 segments of both PCAs. PCoA, posterior communicating artery; PCA, posterior cerebral artery; BA, basilar artery; SCA, superior cerebellar artery; AOP, artery of Percheron.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download