Abstract
Neuropsychiatric systemic lupus erythematosus (NPSLE) involves the central and peripheral nervous system in patients with systemic lupus erythematosus (SLE). It is essential to specify the problems faced by patients with NPSLE because it causes diverse disabilities and impairs quality of life. After performing a comprehensive evaluation, tailored management should be provided for the patient's specific problems. We report here the case of a 30-year-old female with SLE who experienced serious neuropsychiatric symptoms cerebral infarction followed by posterior reversible encephalopathy syndrome and peripheral polyneuropathy. We systemically assessed the patient using the International Classification of Functioning, Disability and Health model as a clinical problem-solving tool and provided comprehensive rehabilitation by focusing on her problems.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease of unknown cause that can affect the skin, joints, nervous system, and/or other organs. Arthritis and skin diseases are the most frequent manifestations of SLE [1], and the main treatments have focused on those symptoms by using medications. Neuropsychiatric systemic lupus erythematosus (NPSLE) can involve the central and/or peripheral nervous system and encompasses a variety of neurological and psychiatric features. Although NPSLE causes diverse disabilities and impairs quality of life, most symptoms and signs are underdiagnosed and undertreated [2]. There are few studies of rehabilitation in patients with NPSLE [3]. We report here a case of NPSLE with severe symptoms. We evaluated multiple facets of the patient using the International Classification of Functioning, Disability and Health (ICF) model, and provided comprehensive rehabilitation management.
A 30-year-old female was diagnosed with SLE at 12 years of age. At around 27 years of age, she experienced several instances of seizures and began taking antiepileptic drugs. On brain magnetic resonance imaging (MRI), diffuse cortical atrophic changes and an old infarct lesion in the left superior parietal cortex were detected. Three years later, she had fever, clonic seizure and loss of consciousness. On T2-fluid attenuation inversion recovery (FLAIR) imaging, a high-intensity signal change was detected in the left temporal and frontal lobes, both occipital lobes, and the midbrain (Fig. 1A). She was also diagnosed with antiphospholipid syndrome. After 15 days of treatment, which included intravenous methylprednisolone, immunoglobulin and anti-epileptic drugs, she regained consciousness and her motor weakness was improved. Follow-up brain MRI showed improvement of the previous lesions, which were suggestive of posterior reversible encephalopathy syndrome (PRES) (Fig. 1B). As she had fever and a urinary tract infection (UTI), the infectious condition following immunosuppressant therapy with cyclosporine was thought to be the cause of PRES. At her first admission to the Department of Rehabilitation Medicine, consistently with the dramatic neurological recovery commonly observed in patients with PRES, she showed mild functional and cognitive impairment. One week after the start of rehabilitation, weakness of the left extremities and fever were noted. Brain MRI showed acute infarction in the right basal ganglia (Fig. 1C). Acute cerebral infarction was considered related to SLE activation due to UTI. One month after treatment in the Department of Rheumatology, she was returned to the Department of Rehabilitation Medicine. We performed an electrodiagnostic study and whole spinal cord MRI to rule out other superimposed diseases, such as neuropathy, transverse myelitis, and myelopathy. The cross-sectional area of the spinal cord from the cervical through the conus medullaris was markedly thin on sagittal images, and no other significant abnormal findings were evident (Fig. 2). The electrodiagnostic study showed severe sensory peripheral polyneuropathy involving the lower extremities.
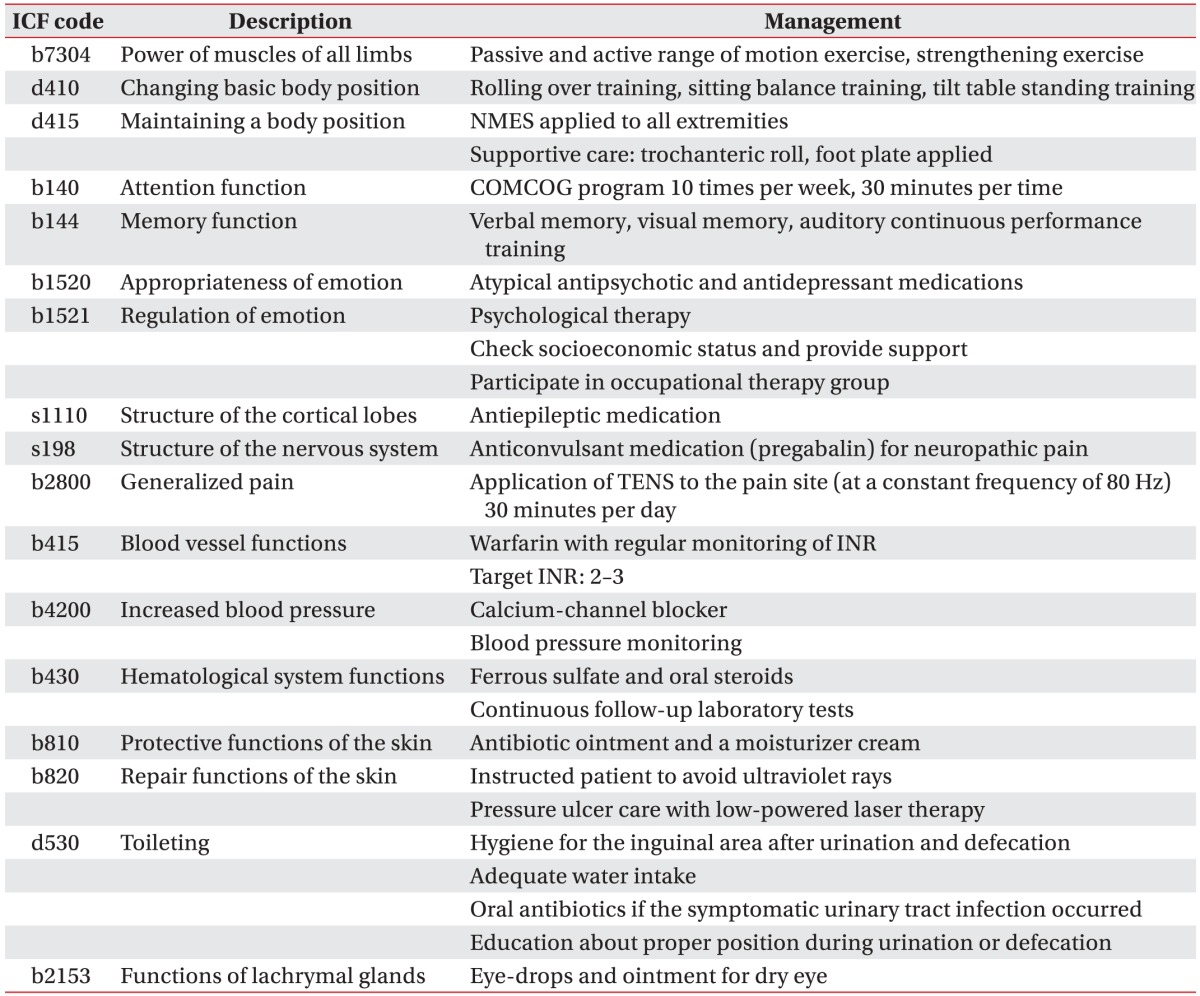
When she was returned to the Department of Rehabilitation Medicine, we specified her clinical problems multiphasically using the ICF (Table 1). After addressing the full spectrum of problems that she had, we comprehensively and rapidly planned how to care for and provide a tailored rehabilitation program to the patient.
Regarding the lower extremity weakness and poor functional level, physical therapy including tilt table standing, trunk muscle-strengthening training and sitting-balance training were administered. To investigate cognitive dysfunction, neurocognitive testing was performed using the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K), and a computerized neuropsychological test was also performed. These showed remarkable impairment in the praxis recall, word list recall, and trail making tests (Table 2). She underwent a therapeutic program using a computer-aided cognitive rehabilitation training system (COMCOG; MaxMedica Inc., Seoul, Korea), which included memory function training and attention training components. As she experienced severe cerebral infarction after UTI, we aggressively controlled her condition to prevent development any further infections as confounding factors. To manage the tingling sensation and neuropathic pain in both of her lower extremities, we prescribed the anticonvulsant, pregabalin. She also experienced psychological problems, including depression and avolition, for which we prescribed anti-depressants and referred her to a psychologist and social work team.
Using the ICF code, our multidisciplinary rehabilitation team could determine the appropriate therapeutic approach to each problem. For example, several interventions were chosen to treat the target intervention "d530 toileting". A physician prescribed antibiotics to prevent UTI, a physical therapist instructed the patient and her parents about proper position during urination or defecation and nurses checked her water intake. This approach allowed us to rapidly achieve the goal. Thus, we believe that use of the ICF code facilitates precise and rapid communication among rehabilitation team members.
After completing the comprehensive rehabilitation program, the patient showed favorable improvements in multiple respects (Table 3).
It is difficult to detect multiple problems in patients with SLE because of the disease's variable activity and severity [1]. We reported a patient with NPSLE with severe involvement of both the central and peripheral nervous system. To our knowledge, this is the first study to evaluate a patient's functional impairment and disability in detail using the ICF model, and attempt to manage the identified issues to improve the patient's quality of life.
As NPSLE causes diverse disabilities and impairs the quality of life, management should be tailored to each patient. The first step of management in patients with NPSLE is to identify aggravating factors, such as hypertension, infection, and metabolic abnormalities. Particularly, infection control is important because an infection can increase SLE disease activity, enabling circulating antibodies to attack blood vessels and increase the risk of cerebral infarction [4]. In progressive cases, immunosuppressive agents and anticoagulation medication can be used for events related to a prothrombotic vascular injury. The next step is to provide symptomatic therapy and tailored, comprehensive rehabilitation.
In clinical practice, the ICF enables precise description of the patient's functional state; illustrates the patient's experience of functioning and the relationship between the rehabilitation goals and appropriate intervention targets; and provides an overview of the resources required to improve specific aspects of human functioning, and the changes in functional state following rehabilitative intervention. This also supports a common understanding of the patient's functioning and communication among rehabilitation team members [5].
Aringer et al. [6] designed ICF core sets for patients with SLE to adequately describe the typical spectrum of problems in functioning. In our case, this core set did not cover all of the problems. For example, our patient had pancytopenia, neuropathic pain, and susceptibility to infection. Thus, modifications specific to each individual patient are required when using the ICF.
Many signs and symptoms of NPSLE can pose a significant diagnostic and therapeutic challenge to physicians and rehabilitation team members. We confirmed the patient's functional status and comorbidities with this team approach using the ICF and designed appropriate interventions to achieve her goals. Thus, we believe that use of the ICF facilitates team communication and enables provision of comprehensive rehabilitation programs.
References
1. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore). 1993; 72:113–124. PMID: 8479324.
2. Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. 2014; 10:338–347. PMID: 24514913.

3. Lee IH, Ryu YU. Physical therapy combined with corticosteroid intervention for systemic lupus erythematosus with central nervous system involvement: a case report. J Phys Ther Sci. 2014; 26:1839–1841. PMID: 25435712.
4. Nencini P, Baruffi MC, Abbate R, Massai G, Amaducci L, Inzitari D. Lupus anticoagulant and anticardiolipin antibodies in young adults with cerebral ischemia. Stroke. 1992; 23:189–193. PMID: 1561646.

5. Rauch A, Cieza A, Stucki G. How to apply the International Classification of Functioning, Disability and Health (ICF) for rehabilitation management in clinical practice. Eur J Phys Rehabil Med. 2008; 44:329–342. PMID: 18762742.
6. Aringer M, Stamm TA, Pisetsky DS, Yarboro CH, Cieza A, Smolen JS, et al. ICF core sets: how to specify impairment and function in systemic lupus erythematosus. Lupus. 2006; 15:248–253. PMID: 16686267.

Fig. 1
Sequential brain magnetic resonance images. A T2-fluid attenuation inversion recovery image shows multiple high-signal-intensity lesions in the left temporal and frontal lobes, both occipital lobes, and the midbrain (A). Fifteen days later, the previously noted lesions were markedly resolved (B). Although the previously noted lesions were completely resolved, acute right basal ganglia infarction (arrowheads, C) was seen on brain diffusion magnetic resonance imaging 1 week later.
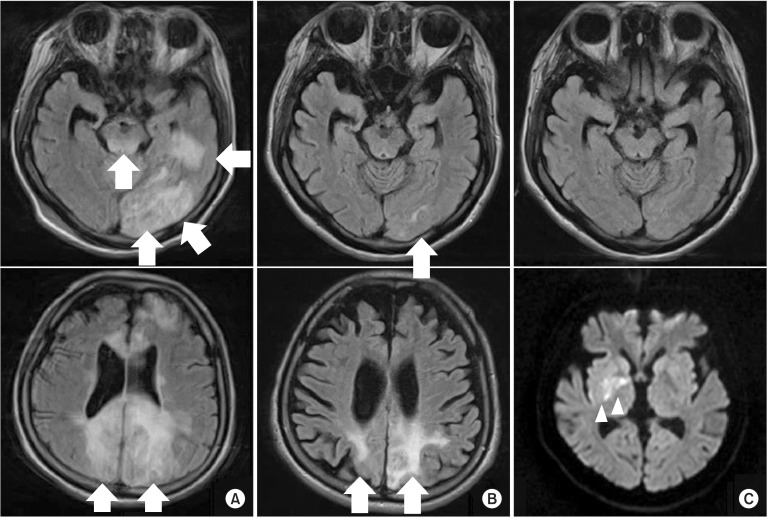
Fig. 2
Whole-spine magnetic resonance image. The cross-sectional area of the whole spinal cord was markedly thin on the sagittal T2-weighted image.
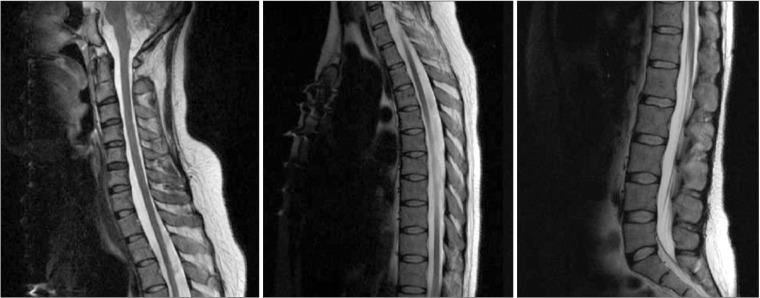
Table 2
Results of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K)
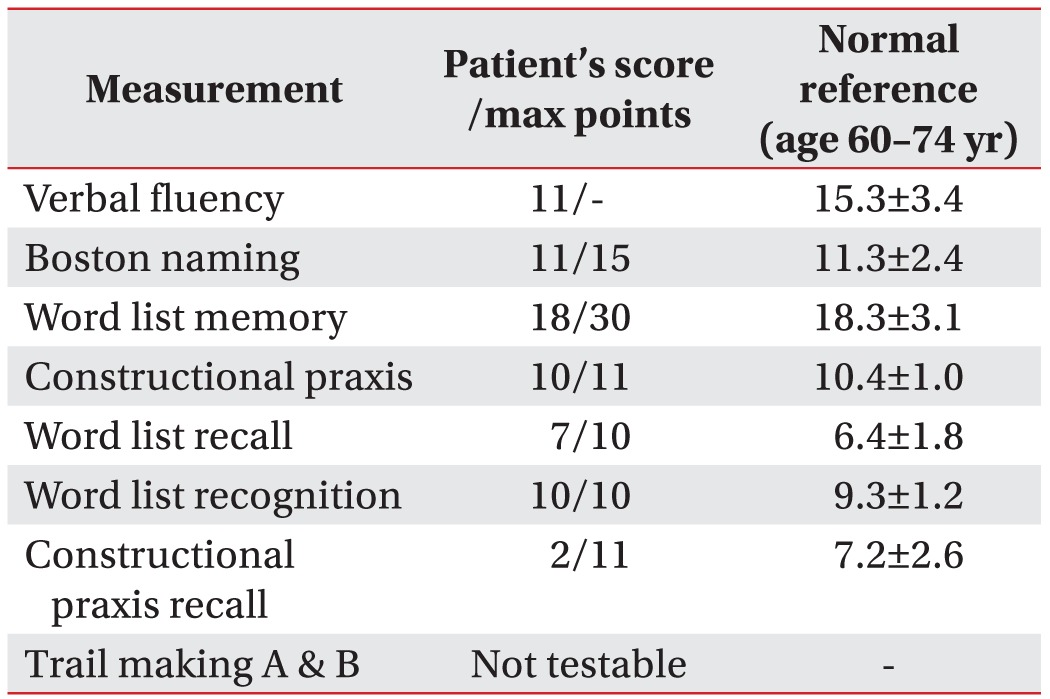
Table 3
Changes in the patient's function and problems before and after rehabilitation therapy
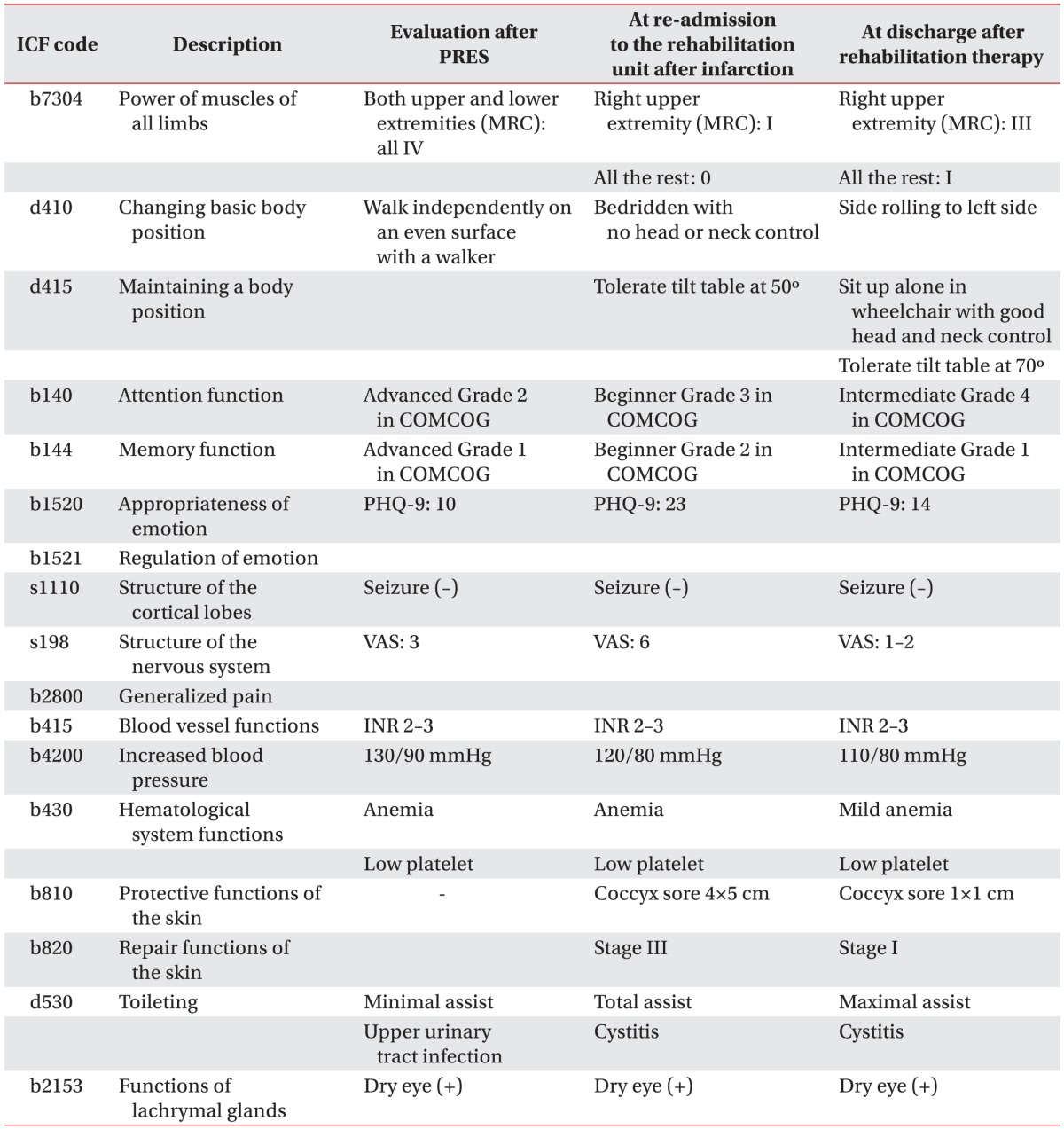
ICF, International Classification of Functioning, Disability and Health; PRES, posterior reversible encephalopathy syndrome; MRC, Medical Research Council; COMCOG, computer-aided cognitive rehabilitation training system (Max-Medica Inc.); PHQ-9, Patient Health Questionnaire-9; VAS, visual analog scale; INR, international normalized ratio.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download