INTRODUCTION
Postoperative motor deficits following neurosurgical procedures result in significant morbidity and mortality rates as well as increased medical costs associated with the extended length of stay and rehabilitation [
1]. For about a decade, intraoperative neurophysiological monitoring has been performed during spine and spinal cord surgery in an attempt to monitor, predict, and mitigate such negative outcomes [
2].
For much of this time, only somatosensory evoked potentials (SEPs) were monitored during spinal cord procedures. However, SEPs do not reflect the functional integrity of motor pathways. The assumption that they do has resulted in a number of so called 'false negative' results, i.e., emergence of postoperative motor deficits despite unchanged intraoperative SEPs [
3].
In recent years, muscle motor-evoked potentials (mMEPs) are also monitored. Many neurosurgeons and orthopedic surgeons now advocate mMEP monitoring for all spinal surgery, since they better predict good postoperative motor outcomes than the use of SEPs alone.
In addition to this predictive power, mMEP data recording benefits from a high temporal resolution; the data may be updated on the order of seconds providing the surgeon with 'real time' information regarding possible surgical trauma. Recent studies have reported the benefits of combining mMEP with SEP monitoring; such benefits have been clearly demonstrated during spinal cord surgery by taking into account the complementary information from two independent systems, these measurements provide increased sensitivity and a reduced risk of false negatives [
45].
Most reports, however, have focused on immediate postoperative motor deficits. Very few studies have follow-ups of any substantial length or reported whether postoperative deficits persisted or were resolved.
Therefore, this study aimed to compare intraoperative changes in mMEPs, SEPs, and both together with the neurological outcomes of spinal surgery, in order to demonstrate any possible advantages of methods using a combined over a single modality. In addition, the correlation between spinal surgery types and intraoperative changes was assessed in our institution.
Go to :

MATERIALS AND METHODS
Subjects
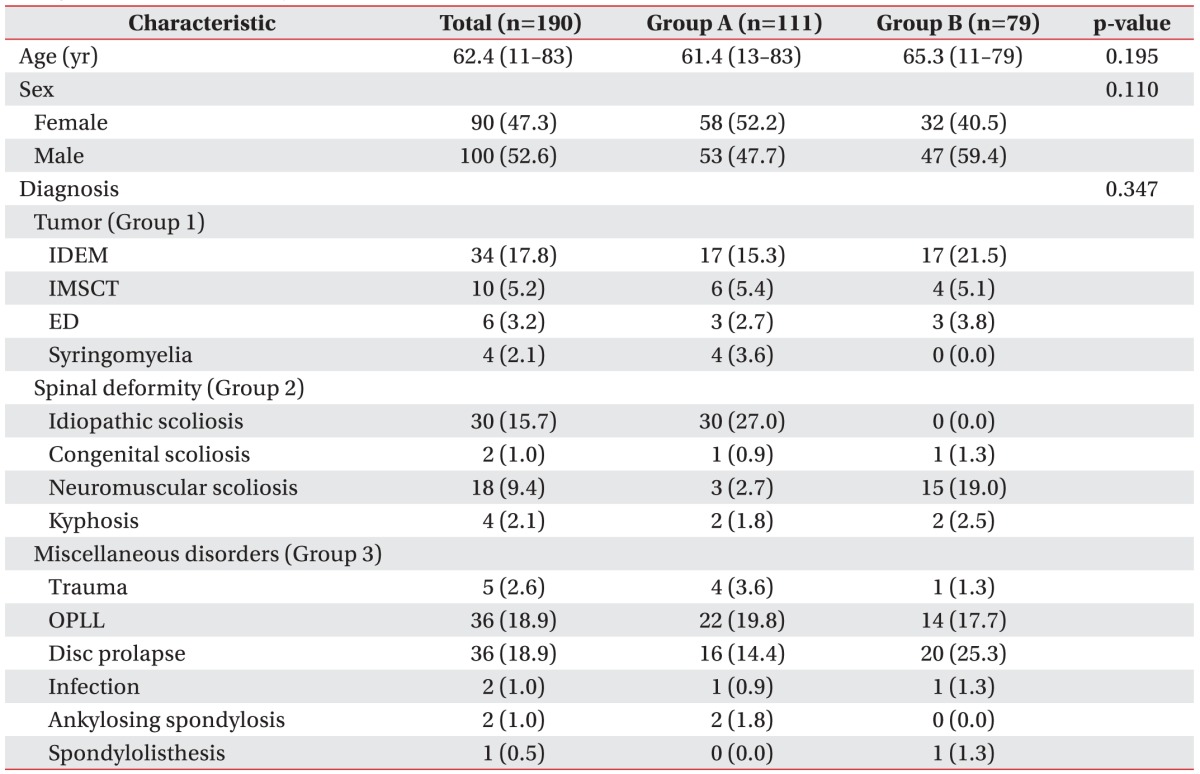
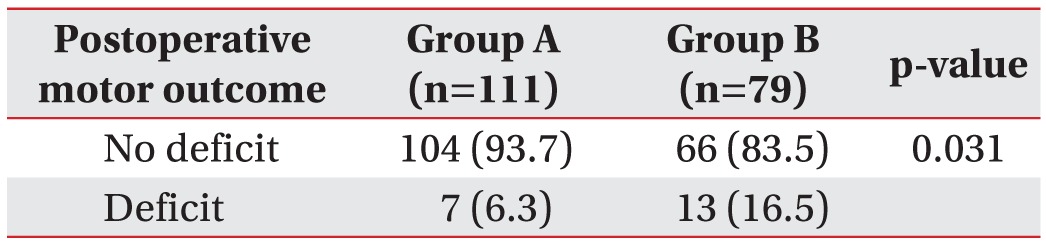
Between November 2012 and July 2014, intraoperative mMEP and SEP monitoring was attempted during 190 spinal operations. All operations were performed by spinal surgeons with extensive experience in the type of spinal surgery conducted. Of the 190 patients, 90 were female. Patients ranged in age from 11 to 83 years (mean, 62.4 years). Prospectively analyzed patient data included medical records, intraoperative monitoring records, operative narratives, anesthesia records, and outpatient clinical notes. Preoperatively, 111 patients were neurologically intact and 79 patients had motor deficits. The baseline characteristics of all participants were listed in
Table 1.
Table 1
Summary of characteristics in 190 patients who underwent spinal surgery (group A, preoperatively motor intact; group B, preoperatively motor deficit)

Procedures
mMEP and SEP monitoring was successful in all patients, who were then subdivided into three groups by diagnosis. The first group (Group 1) comprised 54 patients who underwent operations for spinal cord tumors. The second group (Group 2) was an equally sized group of patients with spinal deformities, and operations were performed in cases of scoliosis or kyphosis. The third group (Group 3), on which other spinal operations were performed, was composed of 82 patients diagnosed with disc herniation, ossification of the posterior longitudinal ligament (OPLL), spinal stenosis, and similar conditions. The study was approved by the ethics committee of the Gangnam Severance Hospital.
Anesthesia
A short-acting muscle relaxant (rocuronium) was used to facilitate tracheal intubation and ventilation; no paralytic agents were subsequently used. Anesthesia was administered intravenously using continuous application of propofol (6–8 mg/kg/hr) and remifentanil (0.15–2 µg/kg/min). Desflurane with nitrous oxide was used as a supplement in 7 surgeries. The level of neuromuscular blockage was assessed by posterior tibial nerve stimulation at the ankle with a train-of-four stimulus to observe muscle twitches.
Direct radial artery pressure, ECG, end-tidal carbon dioxide concentration, pulse oximetry, and temperature were monitored. All patients were kept normothermic with a warming blanket. Normotensive anesthesia was maintained throughout the operation.
Technique for SEP monitoring
Somatosensory evoked potentials (Cascade; Cadwell Industries Inc., Kennewick, WA, USA) were elicited by stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle (intensity 20 mA, duration 0.2 ms, with a repetition rate of 5 Hz). Recordings were performed via surface electrodes from the scalp at C3 (right median nerve stimulation), C4 (left median nerve stimulation), and Cz (right and left tibial nerve stimulation) and from a reference electrode at FPz according to the international 10–20 EEG system.
Technique for mMEP monitoring
Multipulse transcranial electrical stimulation was performed using a commercially available Cascade IOM electrical stimulator (Cadwell Industries Inc.). Transcranial electric motor-evoked potentials were recorded bilaterally from the biceps brachii and abductor pollicis brevis muscles in the upper extremities, and bilaterally from the tibialis anterior and abductor hallucis muscles in the lower extremities using a pair of needle electrodes inserted 3 cm apart in each muscle. Short trains of 5–7 square-wave stimuli of 0.5 ms duration and an interstimulus interval (ISI) of 3 ms were delivered at a repetition rate of up to 2 Hz through needle electrodes placed at C1 and C2 scalp sites. A C1/C2 montage is preferentially used to elicit right extremity mMEPs, while C2/C1 is preferable for left extremity mMEPs. All electrode sites were in accordance with the international 10–20 system.
The stimulation intensity was gradually increased (50 V increments from 250 V to a maximum of 400 V) until mMEP amplitudes were maximized above a minimum of 10 mV.
Electrophysiological monitoring
Electrophysiological monitoring was performed throughout the surgical procedures in our series. Baseline readings were obtained after exposure of the operative zone. Waveforms were analyzed for their peak-to-peak amplitude.
A reduction of more than 50% in, mMEP baseline amplitude elicited by direct cortical stimulation was considered a significant intraoperative change indicative of impairment of functional integrity of the motor pathway. An SEP latency increase of more than 10% from baseline was regarded as significant. If significant EP changes occurred, the surgeons were informed and asked to stop the current surgical intervention. If the EP wave returned to normal, operative activities were resumed. If there was no signal reversal, even after reversing the surgical course and removing any implants, cessation of the procedure was considered.
Neurological examination
The motor status of each patient was evaluated before surgery, within 48 hours postoperatively, and 4 weeks later. Neurological condition was calculated on the Medical Research Council (MRC) scale for muscle strength (with the 10 key muscles of the International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] motor score). The strength of these 10 key muscles ranged from 0 to 5. Thus, the total score ranged from 0 to 50 points on each side. Any reduction in motor score (1 point or more) compared with the preoperative state was considered a neurological motor deficit.
Postoperative motor deficits were assessed in all patients. In the group with immediate postoperative motor weakness, a follow-up was conducted 4 weeks later. Deficits that showed improvement in 4 weeks were characterized as transient, and permanent if they persisted after the 4 weeks. Regardless of deficit type, the intraoperative monitoring data were analyzed and the results were confirmed based on the disease classification of each patient.
Statistical analyses
Data was imported into SPSS ver. 20.1 (IBM, Armonk, NY, USA) for analysis. Based on the characteristics of the variables, we used either an independent-samples t-test or chi-square test to determine significant differences between the preoperative motor intact and motor deficit group in sex, age and diagnoses. Additionally, differences in the rate of postoperative motor deficits between the preoperative status with and without motor impairment were analyzed using chi-square test. Two-sided p-values of <0.05 were considered statistically significant.
Go to :

DISCUSSION
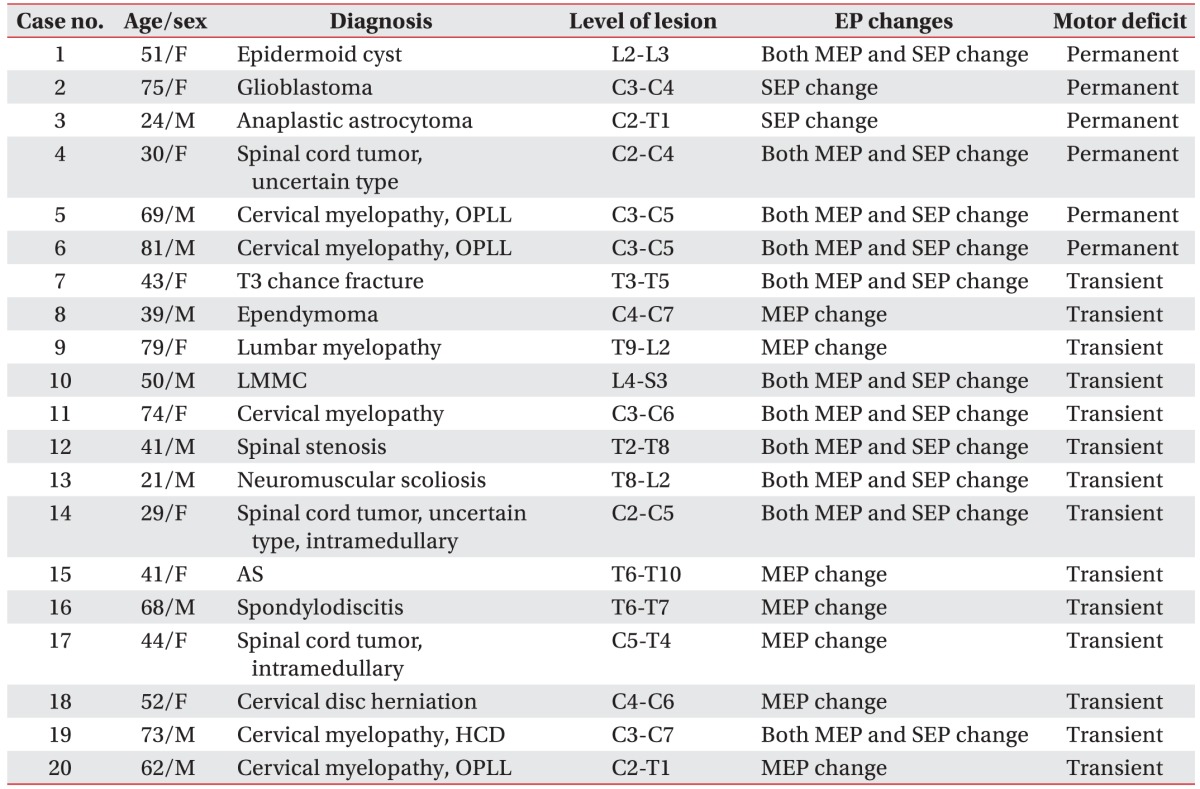
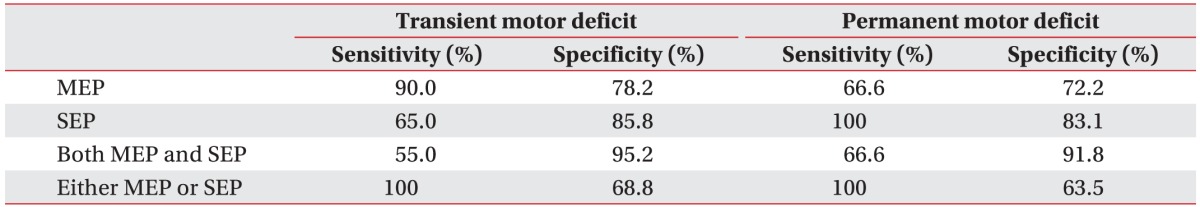
Combined mMEP and SEP monitoring provides a great deal of information for spinal cord surgery. In this study, we monitored 190 patients in all, and could observe EP changes in 73 and neurological weakness in 20. In 18 of the 20 patients, the sensitivities of mMEP changes and mMEP or SEP changes were measured at 90% and 100%, respectively. For mMEP and SEP changes, specificity was 95.2%. In all the patients who showed motor deficits subsequent to spinal deformity surgery and other spinal surgery, mMEP changes were observed. Likewise, changes during each surgery also showed high specificity. In 6 patients, permanent neurological deficits remained, 4 of who had been treated for spinal cord tumors. There were no complaints of headache, seizures, or skin burns postoperatively. The most common concern was direct cortical thermal injury; but over the last 15 years only two cases have been reported [
6]. In a 2002 survey of the literature, published complications included tongue laceration, cardiac arrhythmia, scalp burning at the site of the stimulating electrodes, jaw fracture and awareness [
7].
Although SEP monitoring may be a reliable way to guide spinal surgery, many studies have reported that it is inappropriate to evaluate motor pathway functional integrity, and that it shows high false-negative rates [
8910]. Kundnani et al. [
8] performed combined monitoring on 354 patients undergoing idiopathic scoliosis operations. SEP monitoring in that study showed a specificity of 100% but a sensitivity of 51%. Deutsch et al. [
9] performed SEP monitoring on 44 cases of anterior thoracic vertebrectomy, and showed a high false-negative rate and a sensitivity of 0%. Hilibrand et al. [
10] reported that in cervical operations, SEPs showed a sensitivity of 25% and a specificity of 100%.
Many previous studies have reported that the inability of SEP monitoring to detect motor symptoms and its associated false negatives are because it covers the dorsal sensory tract rather than the ventral motor tract [
11]. Moreover, SEPs are less sensitive in detecting nerve root injuries and thus could miss injuries caused by the process of pedicle screw placement or nerve root traction [
12]. Such problems can be limits of SEPs' use for a standalone monitoring tool. Nonetheless, in this study, SEP monitoring showed high sensitivity and specificity in patients with permanent motor deficits. Garcia et al. [
13] conducted SEP monitoring in 80 patients who had undergone cervical laminoplasty, with sensitivity and specificity results of 100% and 99%, respectively, which implies that SEPs can useful for confirming postoperative motor deficits.
Based on the site of stimulation or recording, intraoperative electrical MEPs can be further classified. For example, MEPs can be recorded over muscle (mMEPs) or over the peripheral nerves (neurogenic MEPs). Some previous studies [
1415] have reported that neurogenic MEPs primarily track sensory, rather than motor, responses and are mediated by the same neural pathways as SEPs. As such, current consensus is against the use of neurogenic MEPs as the sole method of motor pathway monitoring [
1617]. However, mMEPs are the more frequently reported modality of MEP monitoring because of the relative simplicity in generating and recording MEPs. mMEPs are of higher sensitivity than SEPs and can detect spinal motor damage directly. However, Modi et al. [
18] reported that spinal damage might occur due to blood loss during an operation, and thus that mMEP monitoring is inadequate for the sensitive detection of ischemic cord injury. Thus, the two modalities provide complementary information in the process of postoperative monitoring. In this study, we conducted multimodal monitoring by considering 4 cases: SEPs alone, mMEPs alone, mMEPs and SEPs together, and either SEPs or mMEPs alone and analyzed the results.
mMEP or SEP changes were observed in all 20 patients with neurological deficits, and both mMEP and SEP changes were observed in 11 of these patients. mMEP changes alone were observed in 7 patients, and SEP changes alone were observed in 2 patients. In predicting immediate postoperative neurological deficits, mMEP sensitivity was 90%, and sensitivity of either mMEP or SEP change was 100%. Overall SEP sensitivity stood at 65%. Combined mMEPs and SEPs showed sensitivity of 55%. With respect to neurological deficits evaluated after 4 weeks, SEP sensitivity was 100%, but combined mMEPs and SEPs, as well as mMEPs alone, had a sensitivity of only 66.6%. These results might be explained by SEP monitoring showing a low sensitivity early in the process of immediate postoperative monitoring.
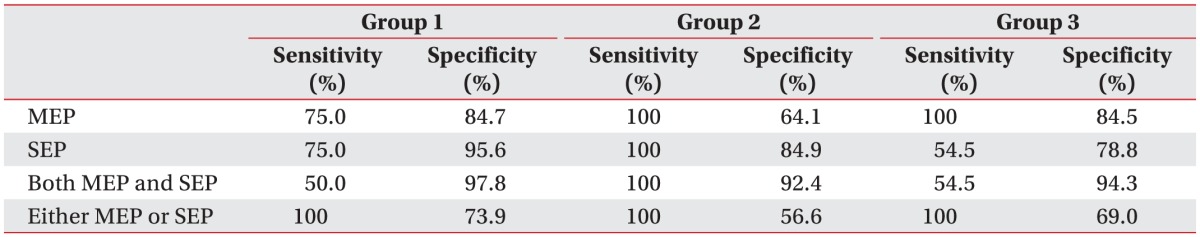
This study analyzed sensitivity in general, but also after specific surgeries, e.g., spinal cord tumor surgery, spinal deformity surgery, and other spinal surgery. In the spinal deformity surgery, combined mMEP and SEP monitoring showed a sensitivity of 100% and a specificity of 92.4%, values that were higher overall than previous reports [
81920]. However, for spinal cord tumor surgery, SEP sensitivity and mMEP sensitivity showed low values overall. For other spinal surgery, SEP monitoring showed low sensitivity, which had the effect of lowering combined mMEP and SEP sensitivity, and also lowered the sensitivity of monitoring in spinal surgery overall. In regard to the especially low sensitivity found in spinal cord tumor surgery, recent studies have reported that initial postoperative deterioration ranged from 18%–24.6% [
2122]. However, Sala et al. [
22] represented that a short-time evaluation was not enough to compare monitored and non-monitored groups, and that follow-ups should be conducted for at least 3 months to observe whether monitoring produces differences in neurological status. Additionally, it may be difficult to induce mMEPs from patients in whom large tumors have caused spinal cord compression or who have had operations or radiation therapies [
23]. In addition, postoperative neurological status is likely to be affected by the surgeon performing the operation, the surgical approach they take, and other underlying diseases not reflected intraoperatively, which might influence the sensitivity of monitoring in other spinal surgery.
Comparison of motor outcomes between the preoperatively intact and deficient motor groups showed significant differences in the rate of postoperative motor deficits, which were higher in patients who had preoperative motor deficits in this study. This may be explained by the vulnerable status of individuals who already had compressed spinal cords amongst the preoperative motor deficit cases. Of the 20 patients in whom neurological deficits occurred, 14 patients showed motor recovery by 4 weeks postoperatively. Thus, such a transient change in muscular strength, namely muscular weakness, was produced by either reversible damage or postoperative pain. In the other 6 patients, their neurological deficits persisted; 4 of these patients showed mMEP and SEP changes, and 2 showed SEP changes alone. As a result, SEP monitoring sensitivity reached 100% for permanent motor deficits related to spinal surgery, and mMEP monitoring sensitivity was 66%. Most of the patients who showed permanent motor deficits had spinal cord tumors. The other 2 patients underwent OPLL surgery. The 4 patients who had spinal tumor surgery showed mMEP and SEP changes, but the 2 patients with intramedullary cervical cord tumors showed SEP changes alone. The surgery for intramedullary tumors involves a myelotomy, which may lead to the loss of SEPs [
2], but mMEPs are frequently unaffected by the loss of SEPs. One patient undergoing this surgery showed a mild (2 point) neurological deficit, which did not change at follow-up. A mMEP change might not be expected with such a mild change. Additionally, it is likely that the pathology and progression of a tumor itself can affect motor deficits on long-term follow-up. The two patients who showed SEP changes alone had glioblastoma and anaplastic astrocytoma. Collectively, our results demonstrated that SEPs and mMEPs have complementary roles in evaluating motor deficits and should be considered together rather than alone.
This study used amplitude variation as a criterion of mMEP change when the baseline decreased by 50% or more [
4242526]. Thus, if the baseline was 80%, it might miss 5 patients who showed permanent neurological deficits, and also increase the false negative rate. Some studies have reported high sensitivities or specificities despite a baseline change of 50% [
1127], but contrarily, setting the baseline at 50% for surgery on 29 patients with cervical kyphosis resulted in mMEPs with a sensitivity of 75% and a specificity of 84% [
25]. In 1,445 cases of anterior cervical surgery with a baseline of 60%, mMEP changes were observed in 267 patients, but neurological deficits were observed in only 2 [
26]. On the whole, a tendency towards excessive false-alarms appears and specificity falls when the cutoff is set between 50%–60%.
In our study, propofol and remifentanil were the primary anesthetics used; desflurane was given as a supplement in only 7 surgeries. mMEP monitoring is more affected by inhalation anesthesia and muscle relaxants, as compared to neurogenic MEPs [
2829]. mMEPs are especially sensitive to the effects of halogenated anesthetics that depress mMEP responses by depressing anterior horn neuron excitability [
30]. This suppression by inhalation agents is dose dependent, has the effect of MEP amplitude reduction [
31], and results in the wide advocation of intravenous over inhalation anesthetics during mMEP monitoring. However, in our 7 operations with added desflurane, no depression in mMEP monitoring was seen.
While some previous studies have analyzed monitoring efficacy subdivided by surgery type, this was the first to analyze sensitivity and specificity in various spinal cord diseases and to also conduct follow-ups on postoperative neurological deficits and later motor recovery. Nonetheless, it has its limitations. The first is that different surgeons performed their operations in different ways and the patients' underlying diseases or conditions were not reflected in the study. Moreover, this study strictly defined 'motor weakness' as even a single point fall on the MRC scale. Therefore, it included even the most mild weakness, which presumably also affected sensitivity and specificity. An additional weakness of our study is that sensory deficits were not checked and we only focused on the motor outcomes. The follow-up, conducted 1 month later, is also a limitation. Additional follow-ups at 6 and 12 months would enable better interpretations of the benefits of SEP and mMEP monitoring. Additionally, this study focused on SEP and mMEP monitoring, but a combination of mMEP and D-wave recording is usually regarded as an optimal way to evaluate the motor pathway in spinal cord surgery [
22], and the application of the absence-presence ground and the epidural D-wave reduces the false positive rate [
2]. In addition, free EMG can be used to monitor the motor tract during spinal cord surgery [
32]. Multimodal monitoring provides surgeons with more complete neurological information, which is expected to provide additional information regarding risk factors, and further enhance sensitivity.
Intraoperative monitoring is often used during spinal surgery. From our data, we can draw conclusions as follow: the sensitivity of SEP monitoring was 100% in the case of spinal deformity surgery. Also, in the case of spinal tumor surgery, SEP monitoring enhanced sensitivity and specificity when used with MEP monitoring. For that reason, the use of SEP can be considerable a good option for spinal cord monitoring and combined SEP and mMEP monitoring should be conducted simultaneously in order to prevent further neurological injury.
In the future, additional kinds of intraoperative monitoring and longer follow-up periods should be conducted in order to better predict postoperative motor weakness.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download