INTRODUCTION
Spasticity is a feature of the upper motor neuron syndrome that has a variety of causes and presentations depending on the location, age, and size of the lesion, following injury to the brain or spinal cord. In stroke patients, spasticity is a major feature of functional impairment, whose incidence after 12 months from the onset is up to 42.2% [
123]. Early treatment is necessary to prevent secondary maladaptation, functional impairment, loss of activity and participation.
Evidence from several randomized clinical trials and meta-analyses demonstrated the significance of botulinum toxin (BTX) [
456]. BTX is a potent inhibitor of acetylcholine release from nerve endings. However, BTX has certain side effects such as dysphagia, pneumonia, and sometimes it can even lead to life-threatening conditions, which are usually consequences of spread of the toxin away from the targeted areas. In addition, the intramuscular injection procedure itself is an invasive procedure with potential to cause bleeding-related complications. Intramuscular injections into deeply located muscles may potentially lead to compartment syndrome.
Patients with spasticity frequently take anticoagulants since many of them have underlying cardiovascular diseases or thrombotic conditions. These patients may be prone to complications related to intramuscular injections, due to their increased bleeding tendency. Despite this increased risk, there are no guidelines for intramuscular injections in patients taking anticoagulants. In order to formulate a guideline, the physiatrists' attitudes and practices across different countries in performing BTX intramuscular injection in patients taking anticoagulants, need to be elucidated with consensus and compromised among BTX injectors globally. As a cornerstone of an international collaboration study [
7], we undertook a questionnaire study across Korea. In Korea, BTX injections for limb spasticity and dystonia are mainly performed by physiatrists. The questionnaire aimed to assess physiatrists' preference for the ideal international normalized ratio (INR) before performing intramuscular BTX injection, individual guidelines used, their experience with bleeding events in anticoagulated patients during the procedure, and their tendency for making provisions.
Go to :

MATERIALS AND METHODS
We administrated a questionnaire survey to physiatrists across Korea. In order to gather subjects who can represent Korean physiatrists' preference, physiatrists who were specialized in various fields, such as cerebrovascular disease, pediatric rehabilitation, musculoskeletal diseases, and cardiopulmonary rehabilitation, were recruited. Participants and members who attended spasticity specialized educational course programs, provided by the Korean Academy of Rehabilitation Medicine and the Korean Society for Neurorehabilitation, were invited to participate in this study. Moreover, open invitations were sent via e-mail or postal services to physicians working in rehabilitation clinics, rehabilitation specialized hospitals or university-affiliated rehabilitation departments. The questionnaires used in this survey were originally designed by West Park Healthcare Centre, Toronto, Canada, and had been previously administered to physiatrists and neurologists in Canada and Turkey [
7]. We suitably translated the questionnaire into Korean. Physiatrists from different rehabilitation departments known to be actively performing BTX injection were invited via postal services or email. Questionnaires were collected from respondents across 7 different provinces in Korea, between April 2014 to July 2014. The original questionnaire study had been approved by the Institutional Review Board of the West Park Healthcare Centre and the Korean version was approved by Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea.
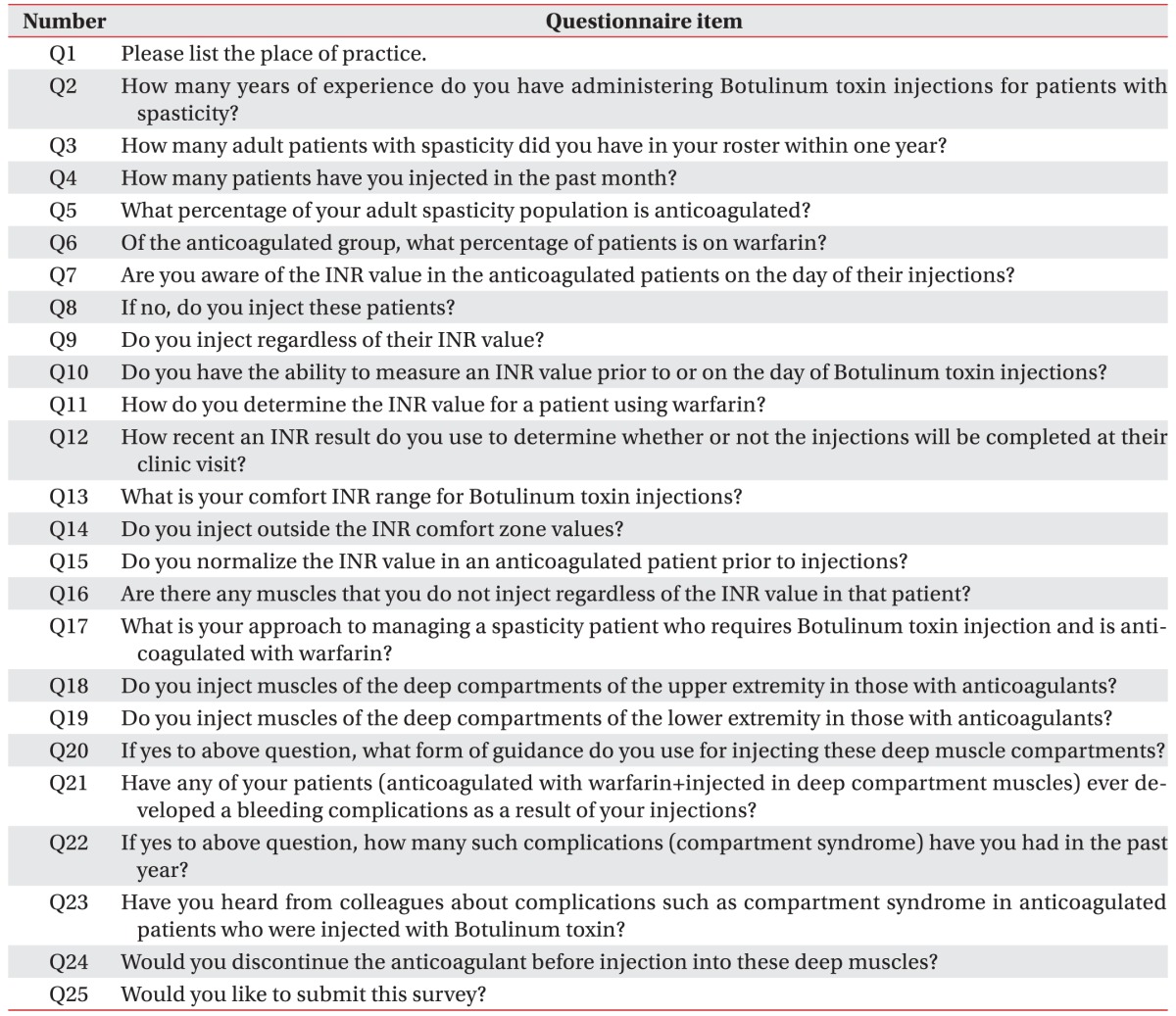
The survey questionnaire consisted of 25 questions (
Table 1), and it was divided into three sections. In the first section (Q1–Q4), physiatrists were asked about demographic details and their career of BTX injection. In the second section (Q5–Q20), physiatrists were asked about their opinion on performing intramuscular BTX injection in anticoagulated patients. These questions focused on whether they checked the INR level prior to BTX injection and which level was considered comfortable for performing the injection. In the third section (Q21–Q24), physiatrists were asked about their tendency to inject into deep muscles and experience of bleeding complications in anticoagulated patients. In addition, specific to this study, we analyzed whether the level of clinical experience with BTX injection could result in different outcomes in making provisions to avoid bleeding complications, and this part was not included in the original survey proceeded by the West Park Healthcare Centre. The respondents with more experience were defined as those who had ≥3 years of experience with BTX injection [
89]. Differences between the two groups were evaluated using the chisquare test. p-value of <0.05 indicated statistical significance. Data analyses were performed with SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Table 1
Questionnaire originally provided from Westpark Healthcare Centre

Go to :

RESULTS
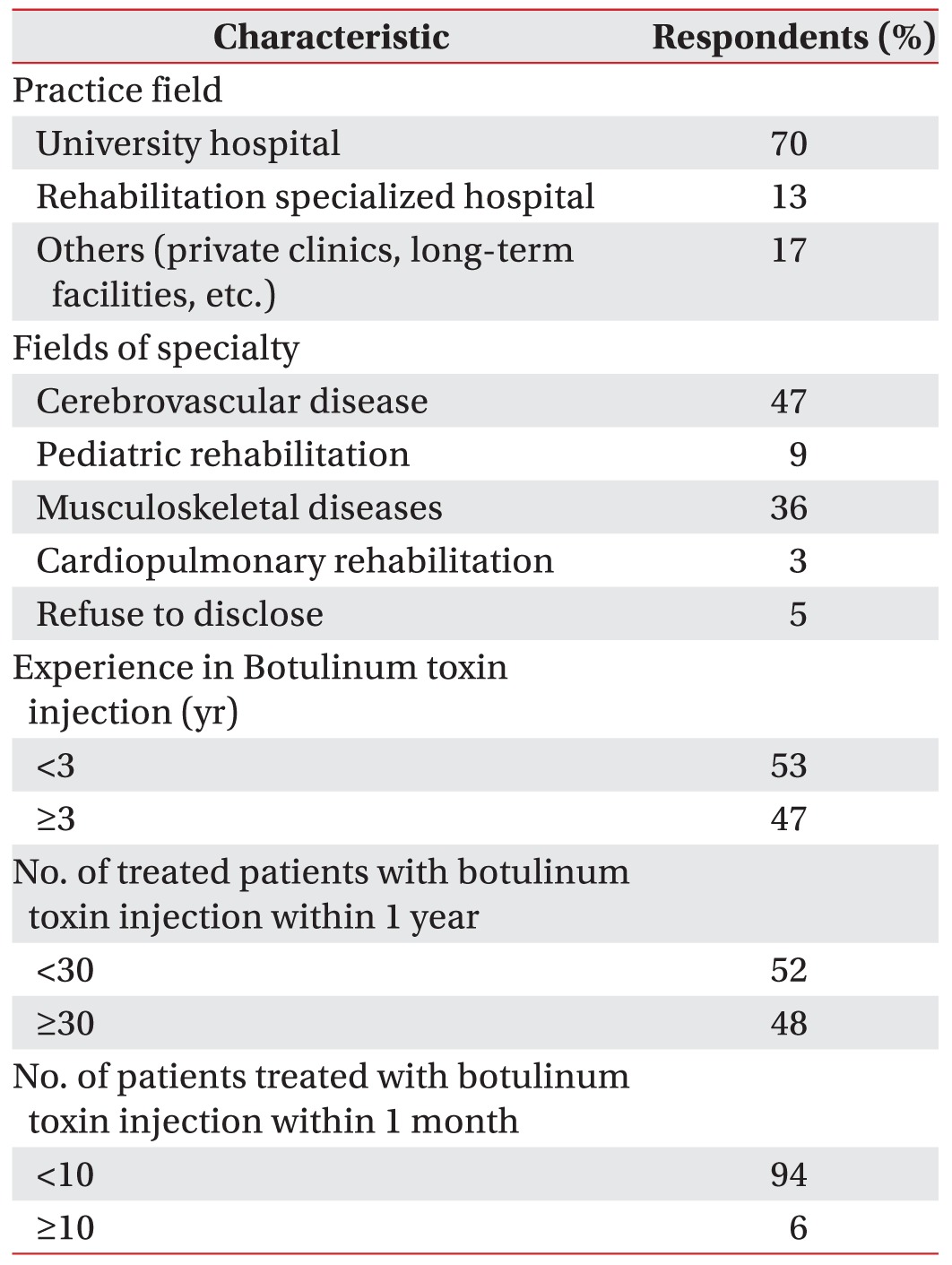
A total of 100 participants willingly agreed to participate in this study. They completed the questionnaire, and returned it to the researchers, yielding a response rate of 100%. Results from the first section of the questionnaire showed that among the total 100 respondents (men 40, women 45, refused to disclose their identity 15), 47 respondents had ≥3 years of experience with BTX injection. Other details regarding the characteristic features of the respondents are presented in
Table 2.
Table 2
Characteristics of the respondents (questionnaire items Q1–Q4)

The second section (Q5–Q20) consisted of questions on the clinical approaches to anticoagulated patients prior to BTX injection. Seventy-seven percent of the respondents replied that 20% of their total spasticity patients took anticoagulants. Many respondents (70%) replied that they were aware of the INR prior to performing the injections in these patients. However, 64% of the respondents replied that they would not inject without knowing the INR level, 13% of the respondents were willing to inject without checking the INR value. It is important to note that as many as 23% of the respondents were uncertain whether they could inject without knowing the INR level due to the lack of existing guidelines (
Table 3).
Table 3
Results of Korean physiatrists' level of INR preference and experience of complications after intramuscular botulinum toxin injection in patients taking anticoagulants
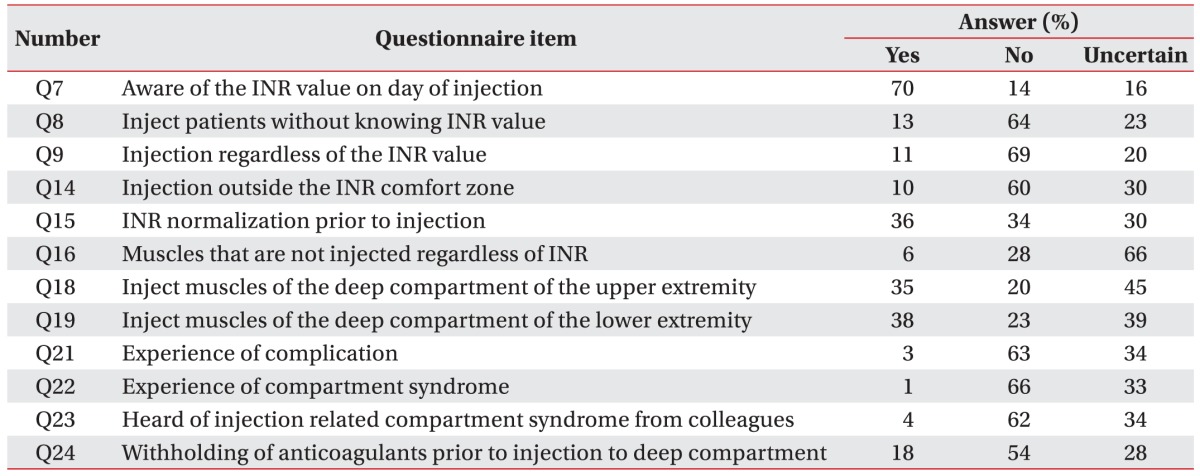

Most of the respondents (78%) had the ability to check the INR prior to the injection, by performing laboratory studies (91%), and only 2% of the respondents replied that they would use a portable coagulometer. While 23% of the respondents checked the INR on the same day the injection was performed, 48% of the respondents used the INR value that was determined 2–7 days prior to the injection to make the final decision regarding their practice. Only 3% of the respondents answered that they would refer to the INR values obtained 8–14 days before the injection, and as much as one-fourth (25%) of the respondents responded that they were uncertain about how recent an INR result should be considered.
An INR range between 2 to 3 was perceived to be ideal by 45% of the respondents. A large number of respondents (41%) replied that an INR lower than 2.0 was the appropriate range to perform BTX intramuscular injection (
Fig. 1). While only 3% of the respondents were willing to perform BTX injection with an INR >3.0, 11% of the respondents replied that they were uncertain about the safe INR range to perform intramuscular injection. In addition, only 8% of the respondents preferred to inject outside their preferred INR safety zone. Further subgroup analysis was performed among the physiatrists who specialized in pediatric rehabilitation, since they have a lesser chance of prescribing anticoagulants to their patients and their preference may be different from that of physiatrists who specialized in adult spasticity. The results showed that the majority of pediatric physiatrists had experience with BTX intramuscular injection in more than 50 patients. While 5 (55.5%) of the respondents thought that the INR safety range should be below 2.0, the majority (88.9%) of the respondents preferred to normalize or withhold anticoagulation before injection and only 1 (11.1%) respondent preferred to inject outside the safe INR range. When these preferences were compared to those of physiatrists who did not specialize in pediatric rehabilitation, there was no significant difference (p=0.91). A unique trait observed in this Korean study was that 36% of the respondents replied that they preferred normalization of the INR to sub-therapeutic levels before injection (
Table 3).
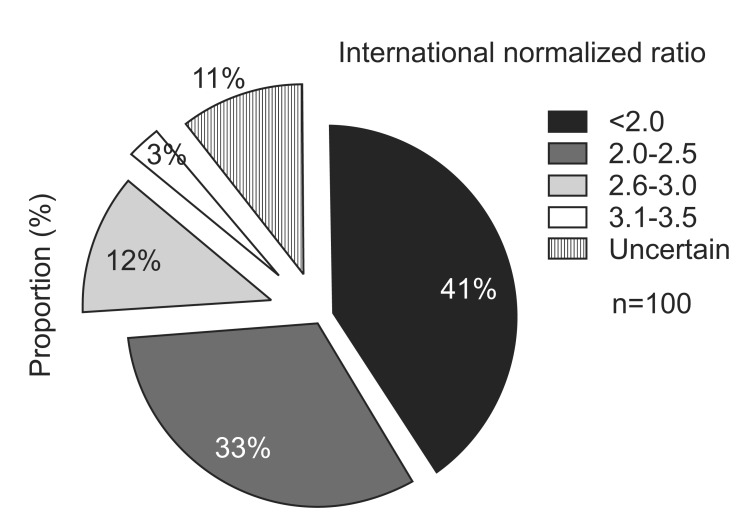 | Fig. 1Pie chart shows Korean physiatrists' preference range of international normalized ratio before intramuscular botulinum toxin injection in anticoagulated patients (questionnaire Q13).
|
Other than controlling the coagulation profile, only 31% of the respondents took certain preventive measures to avoid complications and as many as 69% of the respondents replied that they did not have any standardized protocols for performing BTX injection. The preventive measures suggested by some respondents were application of prolonged compression on the injection site and observation of signs of a bruise or swelling after the injection. Although a small number, some respondents suggested that injection should be avoided and rather they preferred to use oral antispasticity agents, perform conservative physical therapy, or prescribe corrective orthosis in patients at risk of bleeding. Some respondents recommended using ultrasonography during and after the injections to directly visualize the vessels and to check for bleeding.
Results from the third section of the questionnaire showed that 6 respondents replied that they were unwilling to inject BTX into some muscles even with a normalized INR, and these muscles were tibialis posterior, quadriceps, pronator teres, iliopsoas and the tibialis anterior muscles (questionnaire Q16). On the other hand, 28% of the respondents were willing to inject all muscles, regardless of their deep location in anticoagulated patients. The remaining 66% of the respondents were uncertain about which muscles they should avoid even with a normalized INR range. Some respondents (18%) replied that anticoagulants should be intentionally withheld and discontinued prior to BTX injection when injecting into the deep muscles.
When respondents were asked about the type of guidance they would use to approach the deep muscles in patients taking anticoagulants, 36% of the respondents replied that they would use portable electrical stimulation or electromyography (EMG) guidance whereas only 5% of the respondents replied that they would use ultrasonography. As many as 59% of the respondents were uncertain about which guidance tool would be appropriate for performing intramuscular BTX injection in patients taking anticoagulants. Only 3 physiatrists reported to have experienced bleeding complications, and only 1 physiatrist confirmed to have encountered a case of compartment syndrome in his practice in anticoagulated patients in the past year (
Table 3).
The respondents with more experience were defined as those who had ≥3 years of experience with BTX injection. Although respondents with more experience were more willing to inject the deep compartment muscles in anticoagulated patients than those with less experience, they showed no difference in their perceived safe INR range or in making provisions to avoid bleeding complications (
Fig. 2).
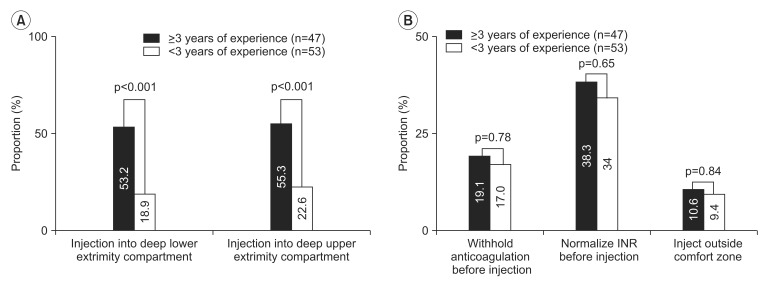 | Fig. 2(A) shows that a larger proportion of experienced physiatrists were willing to inject the deep compartment muscles of the upper and lower extremities than those with less experience. (B) shows the proportion of physiatrists who responded to withhold anticoagulation, normalize the international normalized ratio (INR) and not inject outside the perceived safe range showed no statistical differences between the two groups.
|
Go to :

DISCUSSION
The objective of this study was to determine the preference and tendency of Korean physiatrists in controlling the coagulation profile when performing intramuscular BTX injection in anticoagulated patients. Overall, the results of this study show that considerable practice variability and uncertainty still exists among physiatrists in deciding the INR range at which the injection should be performed, which deep compartment muscles should be avoided, and in the guidance method to avoid bleeding complications. The results revealed that the majority of Korean physiatrists tended to take the INR range into consideration prior to BTX injection and their practice could change according to patients' INR level. Many physiatrists refrained from performing the injection unless the INR was <2.0 and intentionally controlled the INR level to sub-therapeutic levels by discontinuing or lowering anticoagulants, in order to avoid bleeding complications in the deep-lying muscles. This trend was observed irrespective of their level of experience with BTX injection. Results showed that only few respondents reported to have experienced complications related to intramuscular BTX injection in anticoagulated patients. The presence of such highly variable approaches emphasizes the need to formulate a proper international consensus for performing BTX management in anticoagulated patients.
If INR is controlled above the therapeutic range, patients undergoing anticoagulant therapy are at risk of spontaneous bleeding. The major factors associated with bleeding risk are advanced age, intense anticoagulation, and increased number of prescription medications [
10]. Sometimes anticoagulants have been reported to facilitate the onset of an acute compartment syndrome, even in the absence of any trauma event and within the optimal INR range [
1112]. Compartment syndrome may result in ischemia and necrosis leading to irreversible damage [
12]. Therefore, proper INR monitoring before BTX intramuscular injection is essential to minimize bleeding-related complications. The study results showed that most physiatrists were aware of the patient's INR before the procedure, although variability was observed in how recent a tested INR value they would use prior to injection. The most commonly recommended target range for anticoagulation is an INR of 2.0 to 3.0 [
1314].
Once the INR is within the therapeutic range (2.0–3.0), the use of anticoagulants is not associated with an increased risk of hematoma formation or other bleeding complications even in high risk muscles [
15]. A recent study has also reported that the use of anticoagulation did not result in more hematoma formation after BTX injection [
16]. Complications caused solely by injection itself are reported to be very rare, indicating that injection itself is a relatively safe procedure after appropriate safety precautions are taken [
17]. This indicates that lowering the INR to sub-therapeutic range (INR<2.0) is unnecessary.
Strictly controlled INR below the therapeutic range (INR<2.0) can instead result in life-threatening complications. Results of our study surprisingly showed that in order to safely perform BTX injection in anticoagulated patients, majority of Korean physiatrists emphasized on an INR of <2.0, which is the sub-therapeutic range and showed a trend for tapering anticoagulation or even stopping medication to normalize the INR. This is in sharp contrast to Canadian physiatrists' preference regarding the ideal INR range, in which only 10% of the Canadian physiatrists considered an INR <2.0 to be safe [
7]. The survey results from Korea are alarming because lowering INR to the sub-therapeutic level and discontinuation of medication can cause serious thrombotic complications. Results of a study on atrial fibrillation support the notion that the effectiveness of warfarin is reduced when the INR falls to <2.0 and is essentially lost when the INR falls to <1.5 [
18]. The risk for recurrent venous thromboembolism is high if anticoagulation is stopped in the first month after an acute event (40%) [
19]. According to Kim et al. [
20], among the patients who developed stroke in spite of anticoagulation, 89% had INR values lower than 2.0. Similar to the intramuscular injection procedure, a study on needle EMG in anticoagulated patients showed that the theoretical risk of hematoma formation may be lower than the risk of thrombotic events following anticoagulation discontinuation [
21].
However, hematoma and compartment syndrome after EMG have been reported even when INR was in the subtherapeutic range [
22] and BTX intramuscular injection has its own inherent risk. Therefore, precaution and proper standardized protocols are necessary to avoid bleeding complications. One may take other precautions when injecting the deep compartment muscles to avoid bleeding complications rather than avoiding injection or withholding anticoagulation. These precautions may include noting body habitus, which could mask bleeding, and assessing the risk-benefit ratio in each patient before proceeding further [
15]. They may also include the use of a small sized needle [
17], application of firm compression, and when available, use of portable ultrasound to check for hematomas. Use of instrumental guidance such as electrical stimulation or ultrasonography is required as part of standard practice especially in the deep seated muscles [
3]. While electrical stimulation is useful in identifying individual fascicles and locating the motor point, ultrasound allows real-time visualization of target muscles, as well as the surrounding arteries, veins, and bone. Ultrasound also provides the option to apply realtime Doppler during the procedure, thus allowing the operator to avoid vascular structures [
23] and to check for hematoma formation during and after the procedure [
24]. Our results have shown that many Korean physiatrists relied on the electrical stimulation or EMG guidance when injecting the deep compartment muscles. However as suggested recently [
24], portable ultrasonography may be a more appropriate guidance when injecting the deep muscles in anticoagulated patients, rather than electrical stimulation.
This study, which was part of an international collaborative effort to provide information on how injectors worldwide approach BTX treatment in patients under anticoagulation, although it has a simple design, it is clinically significant since the results reflect the clinical practice of physiatrists from different working environments, level of experience, and provinces across Korea. As many as 69% of the respondents replied that they did not have standardized protocols when performing BTX injection in patients taking anticoagulants. This study also showed that BTX injectors require guidelines that state how recent an INR result should be considered before injection, and the upper marginal safe INR limit that allows to perform BTX injection. Lack of guidelines related to the injection of BTX in anticoagulated patients explains the heterogeneity of practices as seen in this survey.
The results of this questionnaire study were not intended to provide direct clinical guidelines. However, through a survey study, basic information on the actual circumstances in which physiatrists across different countries approach these patients is necessary before an international consensus can be made. A similar survey study has been previously carried out with needle EMG [
25]. The results of this study, along with those provided from other countries [
7] would help to lay the basic foundation to establish proper clinical guidelines and consensus on intramuscular BTX injection in patients at risk of bleeding.
This study has several limitations. First, accurate recollection of all prior complications may have been contaminated by recall bias and respondents may have been hesitant to disclose complications despite the anonymous recording of results. Also, the questionnaire did not include information on the INR range at which bleeding complications were observed. Moreover, the respondents many not be truly representative of all the physiatrists' practice in Korea. However, the fact that the questionnaire was collected from several rehabilitation centers across 7 different provinces helped to ensure that the data collected in this study was not deviated to a single center practice and that it included a variety of respondents with diverse levels of experience and training background. But, the present questionnaire did not take into consideration the new generation of anticoagulants such as Pradaxa (Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA) or Xarelto (Bayer Pharma AG, Berlin, Germany), which do not require INR monitoring, and because of the growing trend for using these newer agents, this topic is an issue that needs to be addressed in future studies. Finally, the fact that more physiatrists used electrical stimulation or EMG as guidance during the deep compartment injection may have been affected by the availability of the machine at each center.
The results of this study have shown that some Korean physiatrists may avoid injection in patients who are taking anticoagulants, avoid high risk muscles, or even advise patients to discontinue the medication before the injection. Intramuscular BTX injection is the mainstay of spasticity management [
3]; hence, deferring or limiting treatment may pose additional risk of developing disfiguring contractures. On the other hand, if physiatrists discontinue anticoagulation, then patients may be prone to develop devastating and unforeseen complications.
The number of patients receiving anticoagulation is expected to grow in the future; however, the incongruity in practice as observed in this study indicates that a proper consensus needs to be established in order to optimize BTX intramuscular injection in these patients who are at risk of bleeding complications. The possible risk of hemorrhage in BTX muscle injection is still unclear, and further studies are required to validate the safety margin of INR for BTX injection, thereby providing a consensus for BTX injections in anticoagulated patients.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download