This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To examine whether transcranial direct current stimulation (tDCS) applied over the posterior parietal
cortex (PPC) improves visuospatial attention in stroke patients with left visuospatial neglect.
Methods
Patients were randomly assigned to 1 of 3 treatment groups: anodal tDCS over the right PPC, cathodal tDCS over the left PPC, or sham tDCS. Each patient underwent 15 sessions of tDCS (5 sessions per week for 3 weeks; 2 mA for 30 minutes in each session). Outcome measures were assessed before treatment and 1 week after completing the treatment.
Results
From pre- to post-treatment, there was an improvement in the motor-free visual perception test (MVPT), line bisection test (LBT), star cancellation test (SCT), Catherine Bergego Scale (CBS), Korean version of Modified Barthel Index (K-MBI), and Functional Ambulation Classification in all 3 groups. Improvements in the MVPT, SCT, and LBT were greater in the anodal and cathodal groups than in the sham group. However, improvements in other outcomes were not significantly different between the 3 groups, although there was a tendency for improved CBS or K-MBI scores in the anodal and cathodal groups, as compared with the sham group.
Conclusion
The study results indicated that the facilitatory effect of anodal tDCS applied over the right PPC, and the inhibitory effect of cathodal tDCS applied over the left PPC, improved symptoms of visuospatial neglect. Thus, tDCS could be a successful adjuvant therapeutic modality to recover neglect symptom, but this recovery might not lead to improvements in activities of daily living function and gait function.
Go to :

Keywords: Transcranial direct current stimulation, Stroke, Neglect
INTRODUCTION
Unilateral visuospatial neglect is a neurological disorder that is characterized by a deficit in attention to stimuli on one side of the body [
1]. Although hemispatial neglect can be caused by various different pathological conditions, it arises most frequently after right hemispheric lesions of the middle cerebral artery that damage the parietal-frontal cortical-subcortical network that processes space representation and awareness [
2]. Neglect limits the degree of active participation in rehabilitation programs and is thus associated with poor functional recovery and less successful social reintegration [
3].
As most of the spontaneous recovery after stroke happens in the first month, it is necessary to determine the effects of early but specific interventions for unilateral visuospatial neglect compared to conventional rehabilitation in order to avoid the confounding effect of spontaneous recovery [
4]. Various therapeutic strategies including visual scanning, central cueing, visually displacing prism adaptation, optokinetic, caloric, and vestibular stimulation, neck vibration, and pharmacologic treatments, have been attempted to treat visuospatial neglect [
5]. The rationale for sensory stimulation therapy is based on the hypothesis that spatial representations are made up by the convergence and integration of different afferent inputs as visual, vestibular, and proprioceptive-somatosensory stimuli [
35]. In addition, potent sensory stimulation and training regimes serve to increase sensory input to the damaged hemisphere and redress, at least partially, the balance of activity on the two sides of the brain [
46]. However, these treatments are impractical in the acute or subacute phases of stroke because of the short duration of effects, patient discomfort, and poor patient cooperation.
Recent studies suggested that non-invasive stimulation techniques, i.e., transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), may provide adjuvant tools to promote recovery of function after stroke [
7]. The application of TMS improves impaired contralesional visuospatial processing in neglect patients [
89]. Unlike TMS, tDCS can be used to polarize neural tissue for a longer period of time (i.e., up to a few hours) through the application of weak direct currents that are delivered to the cortex via 2 electrodes placed on the scalp [
10].
The aim of this study was to examine whether tDCS applied over the posterior parietal cortex (PPC) improved visuospatial attention and function in stroke patients with left visuospatial neglect. Outcomes were measured before the first session of the treatment and a week after 15 sessions of treatment.
Go to :

MATERIALS AND METHODS
Subjects
Thirty-two consecutive patients (22 males and 10 females; mean age 62.1 years; range, 39–82 years) admitted for rehabilitation at the department of rehabilitation in Asan Medical Center between October 2013 and December 2014 were recruited according to the following criteria: 1) first ever stroke, 2) left visuospatial neglect, defined as >6.33 mm average deviation from the center line on the line bisection test (LBT) [
11], and 3) diagnosed as right cerebral ischemic or hemorrhagic stroke. Patients were excluded if they met any of the following criteria: 1) severe cognitive dysfunction or aphasia, 2) contraindications for tDCS, such as history of previous seizure, major head trauma, previous brain operation, a metal implant in the brain, or a pacemaker, or 3) systemic disease or ongoing neoplasia. Of the 32 consecutive patients who met the criteria in the recruitment period, 2 were lost to follow-up because of early discharge. Thus, 30 patients were analyzed in this study. All participants provided written informed consent, and our local Ethics Committee approved the study protocol.
Study design
Patients were identified by a number assigned by a centralized computer-generated randomization code. The patients were randomly allocated to 1 of the following 3 groups: 1) the anodal group, 2) the cathodal group, or 3) the sham group. The anodal group received anodal tDCS over the right PPC, the cathodal group received cathodal tDCS over the left PPC, and the sham group received sham tDCS in the same way as active stimulation, but the stimulator was turned off after 30 seconds.
tDCS protocol
Each patient underwent 15 sessions of tDCS. Sessions were 5 times per week for 3 weeks. A direct current was delivered by 2 sets of battery-powered devices named Phoresor II Auto Model PM850 (IOMED Inc., Salt Lake City, UT, USA) using 2 pairs of saline-soaked sponge electrodes (5 cm×5 cm). Stimulation was delivered while the patient was receiving conventional occupational therapy. All patients received conventional physical therapy throughout the duration of the 3 week tDCS protocol. For the real stimulation, a constant current of 2 mA was delivered for 30 minutes. Sham stimulation was performed in the same way as active stimulation, but the stimulator was turned off after 30 seconds. This ensured that subjects could feel the initial itching sensation at the beginning of tDCS and allowed for a successful blinding of the subjects to their stimulation condition [
12]. The tDCS stimulation site corresponded with position P3, which is localized over the left PPC, for the cathodal group; and position P4, which is localized over the right PPC, for the anodal group. Sham tDCS was performed in the same way as for anodal group stimulation, with the stimulation site at P4 and reference electrode over Cz, but the stimulator was turned off after 30 seconds.
These positions are according to the 10/20 electroencephalography system. These locations were previously shown to overlie the PPC in close proximity to the intraparietal sulcus [
11]. The reference electrode was placed over Cz. The choice of Cz was based on previous studies that investigated the effect of tDCS on the primary visual cortex [
13] and parieto-temporal areas. There was no difference in the total amount of rehabilitation time between the 3 groups.
Outcome measures
Each outcome measure was evaluated before the treatment and 1 week after completing the 3 weeks of treatment. Severity of neglect was assessed with the motor-free visual perception test (MVPT), the LBT [
14], the star cancellation test (SCT), and the Catherine Bergego Scale (CBS) [
15].
The MVPT is a 36-item multiple-choice test that evaluates 5 sub-dimensions of visuospatial neglect: visual discrimination, figure-ground discrimination, spatial relationship, visual closure, and visual memory. The parameters that quantify left response behavior (left or right response behavior, raw score, left or right performance behavior, and visual perception processing time) were used. The total score of left response behavior ranges from 0 to 21, and lower scores indicate more severe visuospatial neglect.
The LBT was performed using the method of Schenkenberg et al. [
14]. Twenty lines were drawn on an A4-size sheet of white paper, parallel to the long axis. Eighteen of these lines were organized into 3 sets of 6: 1 set lay primarily on the left of the paper, 1 set lay at the center of the paper, and 1 set lay primarily on the right of the paper. Patients were asked to mark the center points of each of the 18 lines. The deviation from the patients' mark and the true line center was measured for each line, and averaged across all 18 lines for each patient.
In the SCT, 56 stars were mixed with other symbols and presented on an A4-size sheet of white paper. The left and right half of the paper each contained 27 stars, 2 stars were at the midline, and patients were asked to mark all the stars. The number of stars marked on the left side was counted. The score ranged from 0 to 27, with lower scores indicating more severe visuospatial neglect.
The CBS [
15] is a questionnaire based on a direct observation of the patient's functioning by the situations re-created in the clinic in 10 real-life situations, such as grooming, dressing, and wheelchair driving. The score ranges from 0 to 30, with high scores indicating more severe visuospatial neglect.
The Korean version of the Modified Barthel Index (K-MBI) was used to evaluate activities of daily living function. The K-MBI evaluates 10 areas of functioning, and scores indicate the degree of independence. It is scored from 0 (total dependence) to 100 (independence), depending on the level of assistance needed.
Cognitive impairment was assessed using the Korean version of the Mini-Mental State Examination (K-MMSE), and basic motor skills were assessed using Functional Ambulation Classification (FAC).
Statistical analysis
Fisher exact test and the chi-square test were used to compare demographic and baseline clinical variables in the 3 groups (anodal, cathodal, and sham). Change in MVPT, LBT, SCT, CBS, K-MBI, and FAC from initial evaluation to the follow-up evaluation was evaluated using the Wilcoxon signed-rank test. The change in MVPT, LBT, SCT, CBS, K-MBI, and FAC from initial evaluation to follow-up evaluation was compared between the 3 groups using the Kruskal–Wallis test. When significant, the Mann–Whitney U test was performed for pairwise comparison (anodal vs. sham, cathodal vs. sham, and anodal vs. cathodal).
All statistical analyses were performed using SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was considered significant for the Wilcoxon signed-rank test and the Kruskal–Wallis test, and a p-value <0.017 was considered significant for the Mann–Whitney U test.
Go to :

RESULTS
The study included 21 men and 9 women. Four patients had experienced a hemorrhagic stroke, and 26 patients had experienced an ischemic stroke. The hemorrhage was located at the basal ganglia in all patients (n=4). The infarct was located at the following locations: middle cerebral artery territory alone (n=13), middle cerebral artery and anterior cerebral artery territory (n=4), basal ganglia (n=1), middle cerebral artery and posterior cerebral artery territory (n=4), and middle cerebral artery border-zone (n=4). Ten patients were allocated to the anodal group, 10 to the cathodal group, and 10 to the sham group. During the experimental sessions, all patients were blinded to the type of stimulation they received. Baseline clinical characteristics were summarized in
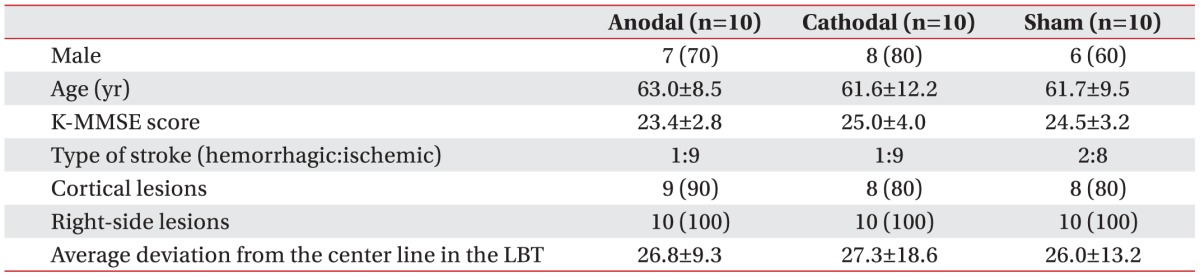
Table 1. At baseline, there was no significant difference in age, gender, lesion site, score on the K-MMSE, number of cortical lesions, number of hemorrhagic strokes, or average deviation from the center of the line in the LBT between the groups. All 30 patients tolerated the treatment without any significant adverse effects, and no seizures were induced.
Table 1
Baseline characteristics of the study patients

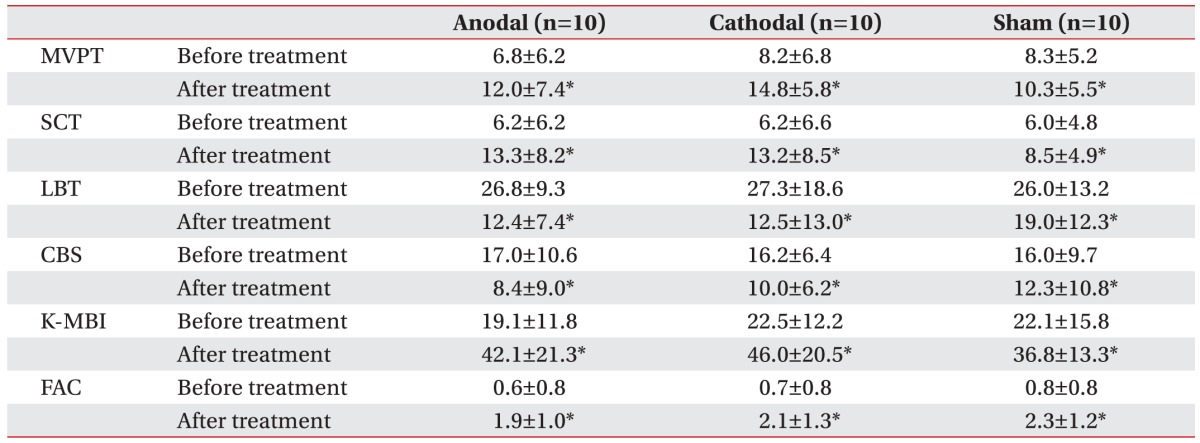
After the 3-week stimulation program, there was improvement on the MVPT, LBT, SCT, CBS, K-MBI, and FAC in all 3 groups (anodal group: p=0.008, 0.005, 0.005, 0.005, 0.005, and 0.017, respectively; cathodal group: p=0.006, 0.005, 0.005, 0.008, 0.005, and 0.012, respectively; and sham group: p=0.004, 0.004, 0.005, 0.011, 0.005, and 0.006, respectively, by Wilcoxon signed-rank test) (
Table 2).
Table 2
MVPT, SCT, LBT, CBS, K-MBI, and FAC scores before and 1 week after treatment in each group

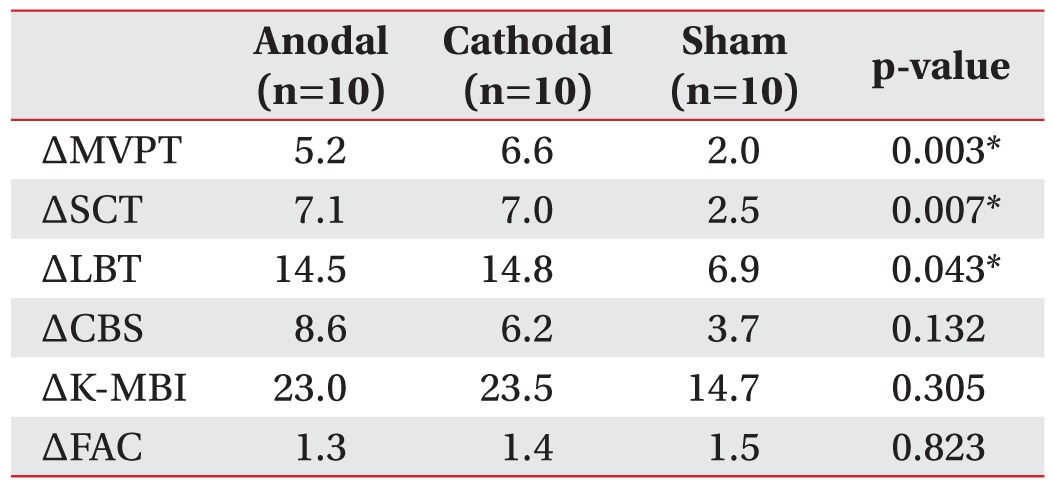
The improvement from the initial evaluation to the follow-up evaluation was significantly different between the 3 groups for the MVPT, LBT, and SCT (p=0.003, 0.007, and 0.043, respectively, by Kruskal–Wallis test) (
Table 3). Post-hoc analysis indicated that the improvement in the MVPT, LBT, and SCT was greater for the anodal group than for the sham group (p=0.016, 0.002, and 0.015, respectively) and greater for the cathodal group than for the sham group (p=0.014, 0.016, and 0.013, respectively) (
Fig. 1). The improvement in the MVPT, LBT, and SCT was not significantly different between the anodal group and the cathodal group (p=0.826, 0.790, and 0.932, respectively). The improvement in the CBS, K-MBI, and FAC was not significantly different between the 3 groups (p=0.132, 0.305, and 0.823, respectively, by Kruskal–Wallis test) (
Table 3).
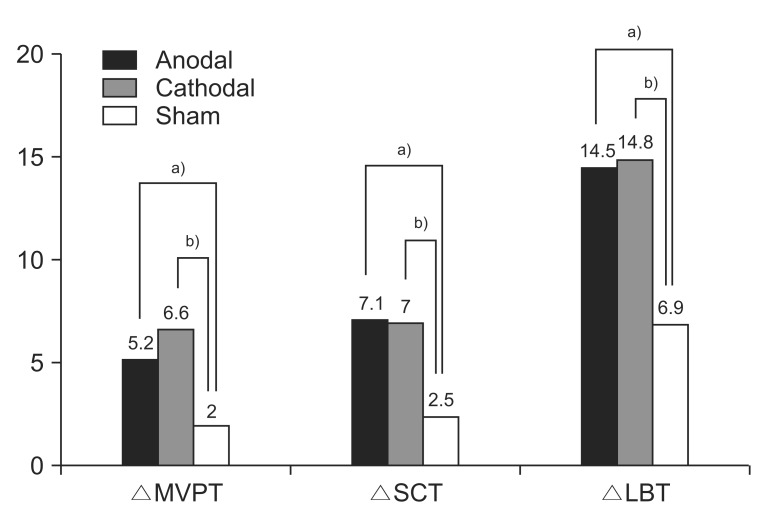 | Fig. 1Improvement in MVPT, SCT, and LBT in each of the three groups. Post-hoc analysis was performed using the Mann–Whitney U test (a)p<0.017, anodal group vs. sham group; b)p<0.017, cathodal group vs. sham group). MVPT, motor-free visual perception test; SCT, star cancellation test; LBT, line bisection test.
|
Table 3
The change (Δ) in neglect and functional status from pre- to post-treatment

Go to :

DISCUSSION
In the present study, we aimed to determine if neglect symptoms and functional ambulation and activities of daily living improved after anodal tDCS to the right PPC or cathodal tDCS to the left PPC during the subacute infarction period. After completing the stimulation program, we observed a greater increase in MVPT and SCT score and a greater decrease in deviation from the line center in the LBT in both the anodal and the cathodal tDCS groups in comparison with the sham group. These results indicated that neglect symptoms were improved following anodal and cathodal tDCS, demonstrating that not only anodal but also cathodal tDCS induces neuronal changes in the PPC. In our present study, improvement of neglect symptoms due to modulation of cortical excitability was observed approximately 1 week after the end of the 3-week stimulation program.
Two previous studies evaluated the effect of tDCS on visuospatial neglect in patients with stroke. Significant improvements in the LBT were observed after both dualmode (right anodal and left cathodal) tDCS and single-mode (right anodal) tDCS, but not after sham stimulation [
16]. Sparing et al. [
10] showed both the inhibitory effect of cathodal tDCS applied over the unlesioned PPC and the facilitatory effect of anodal tDCS applied over the lesioned PPC, and both reduced symptoms of visuospatial neglect. However, these studies were a cross-over design with only one session of tDCS, and were performed in patients with chronic stroke; furthermore, the effects of multiple sessions of tDCS were not evaluated, and the improvement of neglect symptoms was evaluated only immediately after the treatment session. In the present study, neglect symptoms were evaluated at a week after the end of the treatment in stroke patients in the subacute phase.
In the present study, the improvement of neglect symptoms was evident approximately 1 week after the end of the treatment. Although the neural mechanisms associated with these changes are not clearly understood, previous studies reported that tDCS can either hyperpolarize or depolarize the resting membrane potential and recruit larger neuronal populations [
1718]. This effect is mediated by sodium- and calcium-dependent membrane channels and NMDA receptors [
1718]. Additionally, tDCS enhances brain-derived neurotrophic factor secretion and tyrosine receptor kinase B activation, augmenting synaptic plasticity. Therefore, it is possible that anodal and cathodal tDCS-induced activation of these cellular mechanisms and consequent neuronal changes in the PPC occurred in our patients [
19]. In addition, these neuronal changes were present 1 week after finishing the stimulation program. These findings suggested that tDCS promotes brain plasticity after stroke, and affects neglect symptoms. Administering 15 tDCS sessions over 3 weeks might induce relatively longer changes in cortical excitability.
In the present study, cathodal and anodal tDCS were both effective at alleviating neglect symptoms in stroke patients. These findings are in accordance with previous studies using inhibitory (i.e., low frequency) or facilitatory (i.e., high frequency) repetitive TMS to influence PPC function in humans [
8] and cathodal tDCS in cats [
2021]. When the right hemisphere is lesioned, homologous regions of the left hemisphere that normally receive inhibitory projections from the right hemisphere become relatively disinhibited. This possibly generates an unopposed orienting response towards the right side. If the disinhibited intact side is inhibited, attentional bias occurs towards the ipsilesional side of space [
22], suggesting that both parietal lobes exert reciprocal interhemispheric inhibition.
After the 3-week stimulation program, there were no differences in the improvement in the CBS or K-MBI scores across groups, although these scores tended to improve more in the anodal and cathodal groups, as compared with the sham group. Additionally, improvements in the FAC score did not significantly differ between groups. K-MBI score reflects activities of daily living function, whereas FAC reflects gait function. Taken together, our findings suggested that applying anodal or cathodal tDCS over the affected or unaffected PPC, respectively, helped recover neglect symptom, but this recovery did not lead to improvements in activities of daily living and gait function.
In conclusion, applying anodal tDCS over the right PPC or cathodal tDCS over the left PPC improved neglect symptoms, but did not affect activities of daily living function and gait function in patients with subacute stroke. Therefore, tDCS could be a successful adjuvant therapy to improve visuospatial attention in subacute stroke patients.
This study had several limitations, including the small size of the study population. We were unable to compare effects between patients with cortical and subcortical lesions, and with hemorrhagic and ischemic lesions. Also, we did not evaluate the long-term effects of tDCS treatment. Lastly, the sham group consisted of only right anodal sham group. Thus, further studies that address these limitations, including cross-over design to overcome small sample size are needed.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download