Abstract
Objective
Methods
Results
Conclusion
References
Fig. 1
Flowchart of the study. CMS group, core muscle strengthening group; tNMES group, trunk neuromuscular electrical stimulation group; Combination group, core muscle strengthening and trunk neuromuscular electrical stimulation group.
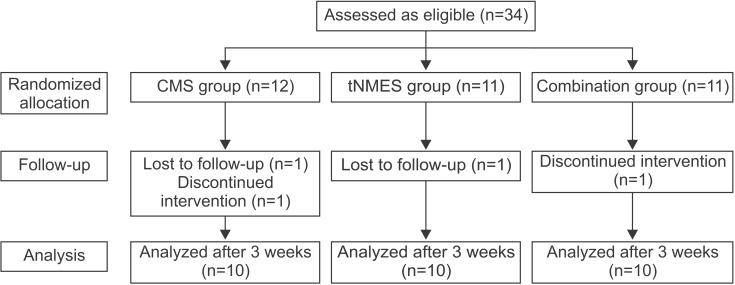
Fig. 2
Surface electodes were placed at 4 sites. Two were on the thoracic erector spinae, and the other 2 were on the lumbar erector spinae.
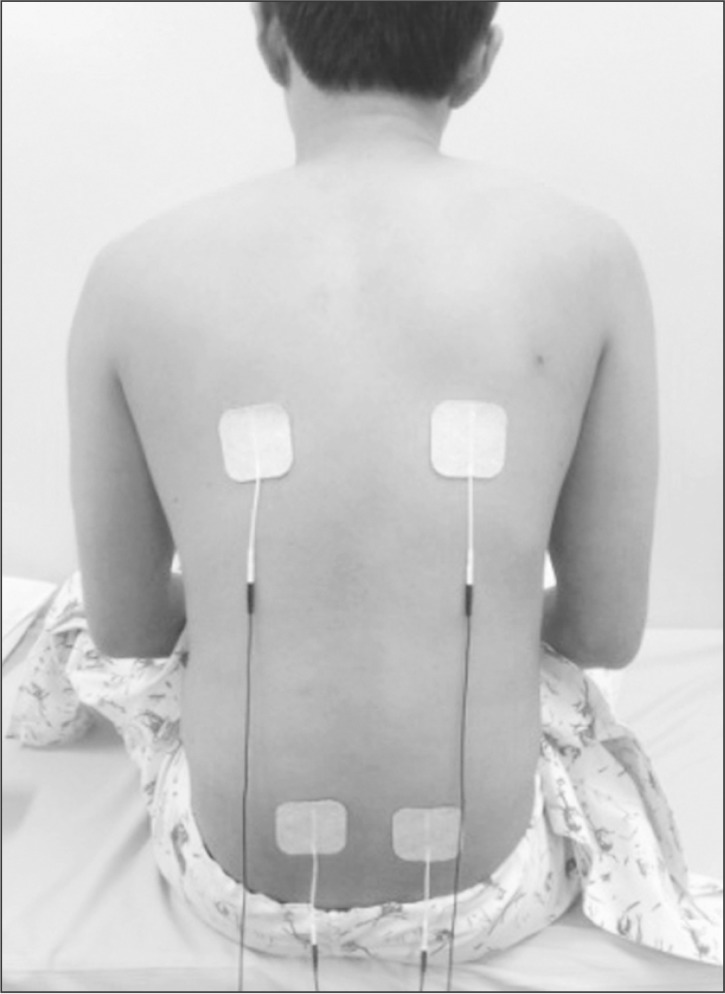
Fig. 3
The core muscle strengthening (CMS) program consisted of (A) the bridge exercise, (B) segmental rotation, (C) the dead bug exercise, (D) plank exercise, (E) belly blaster, (F) bird dog exercise, (G) side plank exercise, and (H) side bridge exercise.
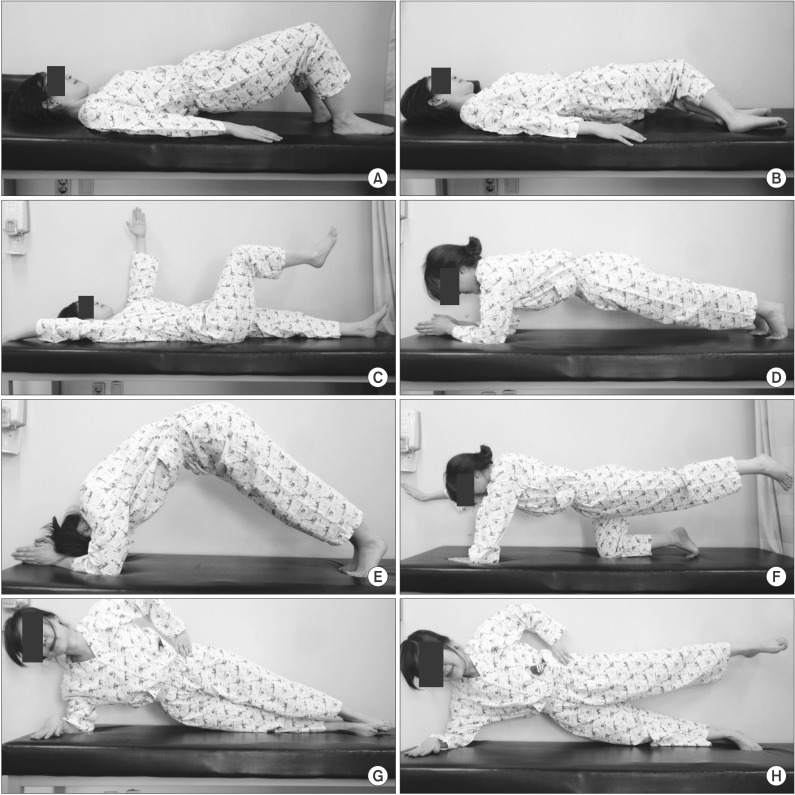
Fig. 4
The comparison of improvements in K-BBS, PASS, TIS, and K-MBI among 3 groups. CMS group, core muscle strengthening group; tNMES group, trunk neuromuscular electrical stimulation group; Combination group, core muscle strengthening and trunk neuromuscular electrical stimulation group; K-BBS, Korean version of Berg Balance Scale; PASS, total score of postural assessment scale for stroke patients; TIS, Trunk Impairment Scale; K-MBI, Korean version of Modified Barthel Index. a)p<0.05 by the Kruskal-Wallis test comparing CMS, tNMES, and combination groups, b)p<0.025 by the Mann-Whitney U-test with the Bonferroni correction to compare CMS and combination group, c)p<0.025 by the Mann-Whitney U-test with the Bonferroni correction to compare tNMES and combination group.

Fig. 5
Correlation between ΔK-MBI and (A) ΔK-BBS, (B) ΔPASS, (C) ΔTIS, (D) Δstatic sitting balance subscale of TIS, (E) Δdynamic sitting balance subscale of TIS, and (F) Δcoordination subscale of TIS. K-BBS, Korean version of Berg Balance Scale; PASS, total score of postural assessment scale for stroke patients; TIS, Trunk Impairment Scale; K-MBI, Korean version of Modified Barthel Index.
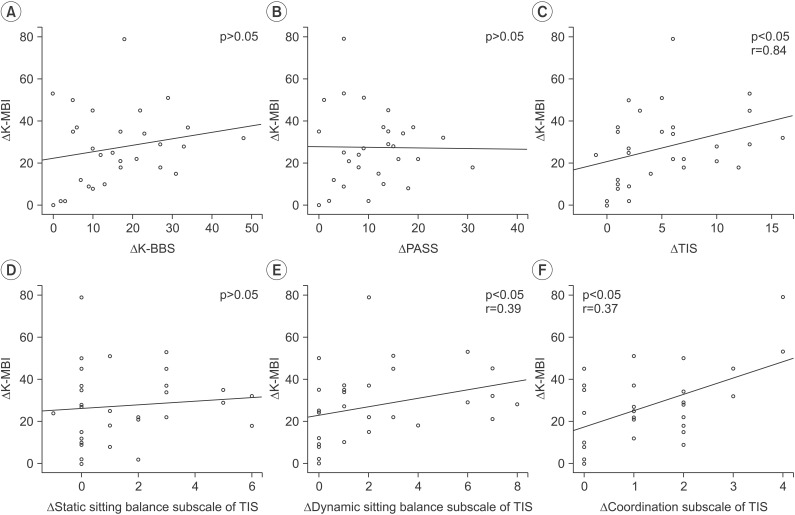
Table 1
Baseline characteristics of patients
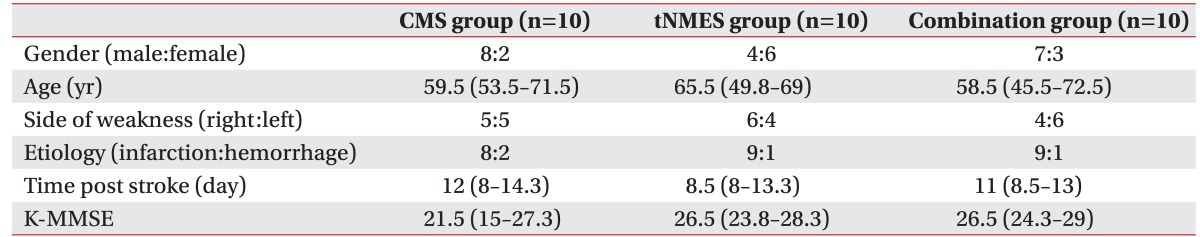
Values are presented as number of patients or median (interquartile range).
CMS group, core muscle strengthening group; tNMES group, trunk neuromuscular electrical stimulation group; Combination group, core muscle strengthening and trunk neuromuscular electrical stimulation group; K-MMSE, Korean version of the Mini-Mental Status Examination.
Table 2
K-BBS, PASS, TIS, and K-MBI before and after treatment in each group
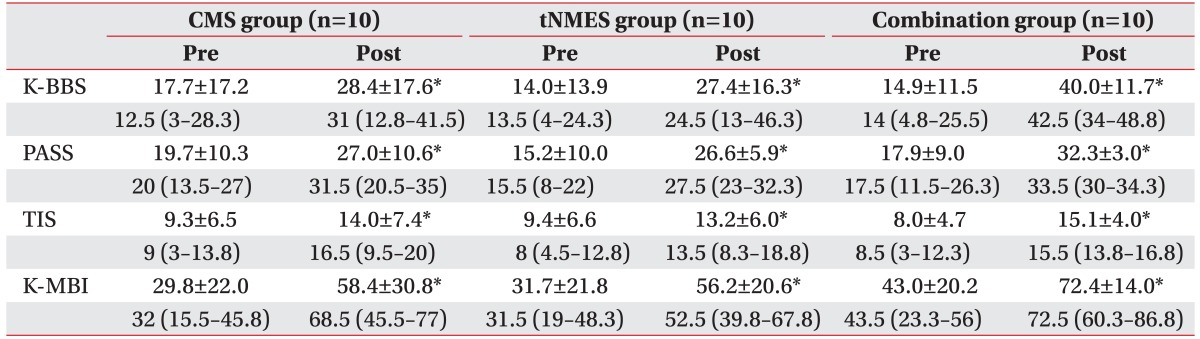
Values are presented as mean±standard deviation or median (interquartile range).
CMS group, core muscle strengthening group; tNMES group, trunk neuromuscular electrical stimulation group; Combination group, core muscle strengthening and trunk neuromuscular electrical stimulation group; K-BBS, Korean version of Berg Balance Scale; PASS, Total score of postural assessment scale for stroke patients; TIS, Trunk Impairment Scale; K-MBI, Korean version of Modified Barthel Index.
*p<0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download