Abstract
Methods
We performed a randomized controlled trial in 33 patients with brain injury with ankle dorsiflexor weakness (muscle power ≤grade 2). Both groups continued conventional customized physical therapy, but the patients in the foot splint group were advised to wear a resting foot splint for more than 12 hours per day for 3 weeks. The data were assessed before and 3 weeks after the study. The primary outcome was the change in ankle dorsiflexion angle after 3 weeks.
Results
Before the study, there were no differences between groups in gender, age, time post-injury, brain injury type, initial edema, spasticity, passive range of ankle dorsiflexion, Fugl-Meyer score (FMS), or Functional Ambulation Classification. A significant improvement in ankle dorsiflexion angle, and FMS was found after 3 weeks in both groups. The splint group showed more spasticity than the control group after 3 weeks (p=0.04). The change of ankle dorsiflexion angle, foot circumference, spasticity, and FMS after adjusting initial value and spasticity were not significantly different between the 2 groups.
Conclusion
Wearing a resting foot splint for 3 weeks did not affect joint mobility in patients with subacute brain injury regularly attending personalized rehabilitation programs. Further studies of larger sample sizes with well controlled in spasticity are required to evaluate the effects of the resting foot splint.
Joint contractures are characterized by restricted passive range of motion, due to either limited extensibility or increased stiffness of the soft tissues overlying the joints, such as periarticular structures and muscles [1]. They represent a common complication of many neurological and musculoskeletal conditions, and cause functional disturbances in joint function, reduce movement ability, and affect daily activities. People with brain injury due to stroke, traumatic brain injury, hypoxia or infection commonly experience significant damage to central motor pathways, and acute paralysis often occurs. The acute paralysis leaves the affected muscles and joints immobilized. Immobilization leads to reduction of longitudinal tension in muscles, which is the basis for muscle contracture [2]. Reduced activity or immobilization due to paresis results in soft-tissue contractures, and changes in muscle contractile properties further aggravate motor impairment, leading to increased spastic paresis [34]. Consequently, brain injury-induced weakness and immobilization frequently result in ankle plantar flexion contractures.
A resting foot splint, also known as pressure relief ankle-foot orthosis (AFO), is generally considered to be effective in preventing the ankle plantarflexion contracture caused by reduction in muscle longitudinal tension. It is easy to apply and can be worn for prolonged periods of time, when the patient is not engaged in exercise. However, to date no studies have evaluated the efficacy of this medical device in patients with brain injury. The objective of our present study was to assess whether a resting foot splint could prevent ankle plantarflexion contracture; in addition, the secondary objective was to examine the effect of this splint on spasticity and foot edema.
Individuals who met the following recruitment criteria were enrolled in the study: 1) first brain injury within the last 3 months; 2) an ankle dorsiflexion power of ≤grade 2; 3) a Functional Ambulation Classification (FAC) of ≤2; 4) an absence of ankle contracture, defined as a dorsiflexion of the affected ankle of <10°, as compared to the intact ankle; and 5) the absence of motor deficiencies prior to the brain injury. Exclusion criteria for participation in this study were: 1) previous ankle surgery in the symptomatic lower limb; 2) previous lower limb trauma that had caused structural imbalance (e.g., ankle fracture); 3) osseous abnormalities of the ankle (e.g., anterior or posterior tibiotalar osteophytes) in the symptomatic lower limb; 4) inflammatory arthritis (e.g., ankylosing spondylitis); 5) peripheral neurologic disorders (e.g., Charcot-Marie-Tooth disease); and 6) injury or pathology of the feet or any condition that, in the opinion of the investigators, could interfere with the findings of the study.
All patients underwent initial treatment for brain injury in the departments of neurology and neurosurgery at our institution, and were then transferred to the department of rehabilitation medicine in the subacute period (1–4 weeks after onset). All patients were diagnosed with brain injury by axial T2-weighted magnetic resonance imaging or computed tomography, and this diagnosis was confirmed by a neurologist or neurosurgeon and a radiologist. Asan Medical Center, University of Ulsan College of Medicine's Institutional Review Board approved the study, and all participants provided written informed consent.
For each patient subject, the following information was recorded: sex, age, time after brain injury, brain injury type, initial foot circumference for edema, spasticity, passive ankle dorsiflexion angle, Fugl-Meyer score (FMS), and FAC. The participants were randomized into 2 groups, i.e., a control and an intervention group. A random table was used for subject assignment to these groups. Both groups received conventional customized physical therapy and gait training 6 days a week for 3 weeks. Data collection and interventions were administered by an experienced qualified physical therapist.
The participants in the intervention group received a customized resting foot splint for the paretic foot. All customized resting foot splints were manufactured from polypropylene with a foot shell, a calf shell and a covering fabric. The splint covered the full length of the foot plantar aspect and the lower leg posterior aspect, maintaining the ankle at a 90° angle without ankle joint. Anterior straps with D rings secured the leg into the splint. Participants were advised to wear the foot splint for >12 hours per day for 3 weeks. The control group received the same therapy except for the resting foot splint. Outcome measures were performed at baseline and after 3 weeks. The primary outcome was the change in the ankle dorsiflexion angle evaluated by aligning a goniometer with the axis of the leg and the lateral side of the plantar surface of the foot with the knee fully extended. The secondary outcome measures were change in foot edema (measured by foot circumference 5 cm proximal to the 1st metatarsal head), spasticity (Modified Ashworth Scale [MAS]), FMS for the lower extremity and FAC [56].
The lower extremity FMS is an examination designed to measure the presence of synergistic and isolated movement patterns, reflex patterns, and coordination (0, lowest score; 34, highest score). The FAC categorizes the patients according to the basic motor skills necessary for functional ambulation (0, absolute walking incapacity, even with external help; 5, normal ambulation). The reliability of all these assessment tools is well documented.
The data were analyzed using the Statistical Package for Social Sciences software (SPSS ver. 18.0; SPSS Inc., Chicago, IL, USA). A Mann-Whitney test was used to analyze between-group differences in age, days after injury, initial foot circumference, spasticity, ankle dorsiflexion angle, FMS, FAC and foot circumference, spasticity, ankle dorsiflexion angle, FMS, and FAC after 3 weeks. A chisquare test was used for comparing the sex ratio and the type of brain injury between the 2 groups. A Wilcoxon signed-ranks test was used to determine the statistical significance of intra-group differences after a 3-week intervention. Changes in foot circumference, spasticity, ankle dorsiflexion angle, FMS, and FAC between the groups were compared using a univariate general linear model. Statistical significance was defined as p-values <0.05.
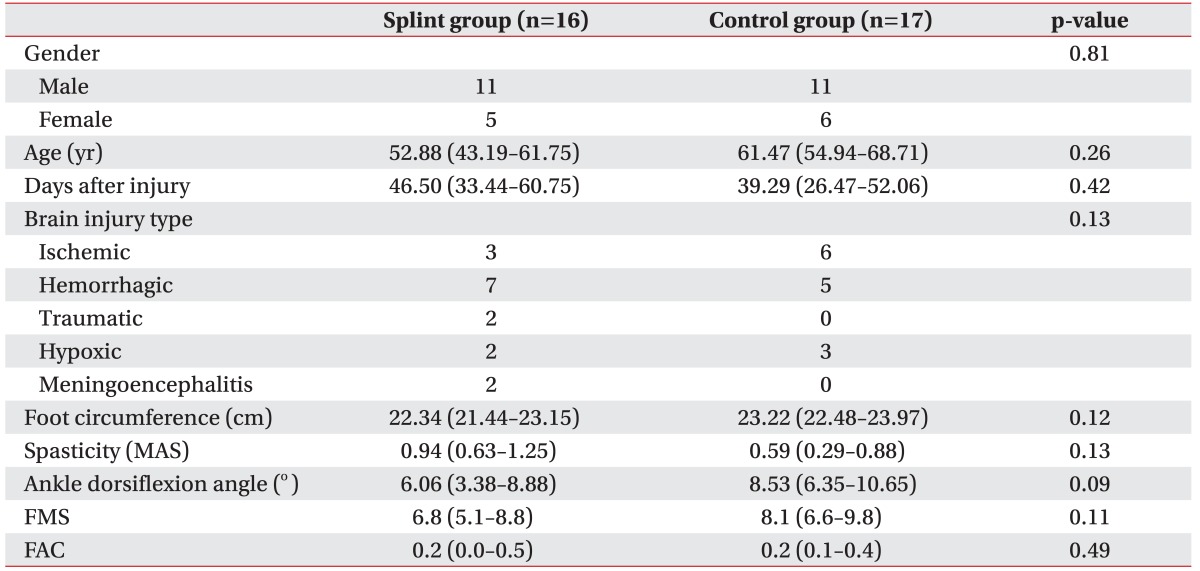
Thirty-three patients were enrolled and randomized into 2 groups (16 in the splint and 17 in the control group). The mean time from brain injury onset to referral to the trial was 42.8 days: 46.5 days (95% confidence interval [CI], 33.4–60.8) for the resting foot splint group and 39.3 days (95% CI, 26.5–52.1) for the control group. Most patients had ischemic or hemorrhagic brain injury. The groups were well matched for gender, age and brain injury types. The initial ankle dorsiflexion angle was 6.06 (95% CI, 3.4–8.9) in the foot splint group and 8.53 (95% CI, 6.4–10.7) in the control group. The other parameters (initial foot circumference, spasticity, ankle dorsiflexion angle, FMS, and FAC) were comparable between the 2 groups (Table 1).
The intra-group and between-group comparisons at 3 weeks were presented in Table 2. Both the foot splint and the control group showed significant increments in the ankle dorsiflexion angle (p=0.02 and p=0.04, respectively) and in FMS (p=0.02 and p<0.01, respectively) in the Wilcoxon signed-ranks test. While FAC was not significantly improved in the foot splint group (p=0.32), it was significantly improved in the control group (p<0.01). The foot circumference and the spasticity did not show significant differences after the 3-week trial in both groups. There was no difference between the 2 groups in baseline spasticity, but the splint group showed more spasticity than the control group after 3 weeks (p=0.04).
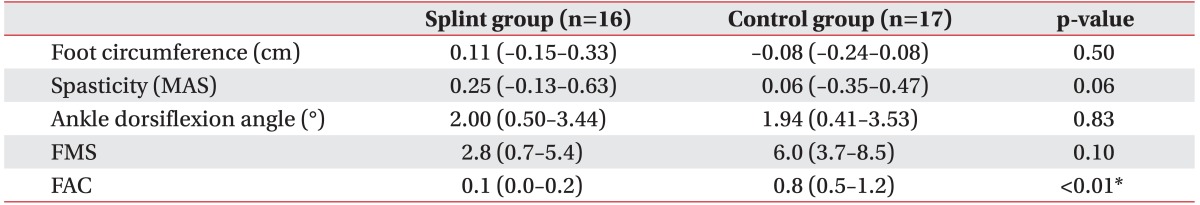
The primary and secondary outcomes were summarized in Table 3. The mean ankle dorsiflexion angle at baseline was comparable in the 2 groups (p=0.09). After 3 weeks, even though both groups showed significant improvement in ankle dorsiflexion angle, after adjusting for the initial value and spasticity at 3 weeks, there was no statistically significant difference between the groups in ankle dorsiflexion improvement (p=0.83). There were also no differences in the change of foot circumference (p=0.50), spasticity (p=0.06) and FMS (p=0.10). The patients in the control group had a significantly better recovery in the FAC score (p<0.01).
Joint contractures after central nervous system injuries have been viewed as occurring through the immobilization of the affected limb induced by muscle paralysis [178]. After acute phase of muscle contracture [91011121314], in the subacute and chronic stages of spastic paresis, the emergence of muscle overactivity becomes an additional mechanism of contracture, superimposed on immobilization, which leads to chronic aggravation of contractures [15]. Contractures are a common complication after stroke [16]. Even if the patients recover from the weakness, a persistent joint deformity, especially due to ankle contractures, limits the performance of functional tasks and is associated with pain, pressure ulcers, and unsightly deformities [1718]. Passive movements, stretching and strengthening exercises, neuromuscular electrical stimulation, and splints are widely used to prevent lower extremity contractures [219]. Despite the wide belief, few studies have actually confirmed the efficacy of these treatments.
In 2013, a Cochrane systemic review found only 16 studies of passive movements for the treatment and prevention of contractures. Only 2 of these reports were regarded as appropriate for inclusion in the analysis, of which only 1 measured joint mobility. In that study, Harvey et al. [1] found a small but statistically significant effect of motion on the ankle dorsiflexion angle range after 6 months of passive movements in patients with spinal cord injury. However, the Cochrane system concluded that the evidence is insufficient to permit any firm conclusions about the effectiveness of passive movements for joint mobility, spasticity or pain [7]. Katalinic et al. [20] reported that stretching does not have clinically important effects on joint range of motion in people with neurologic and non-neurologic conditions in the Cochrane and other systematic reviews. No study to date has explored the effect of neuromuscular stimulation in preventing ankle contracture.
Robinson et al. [21] conducted a randomized trial for 4 weeks. The patients in 1 group wore a splint on the affected ankle at plantar grade 7 nights per week, while the others stood on a tilt table for 30 minutes with the ankle at maximum dorsiflexion, 5 times per week. By week 4, the night splint group had the same amount of ankle dorsiflexion as the tilt table group. However, there was no control group, so it is not clear if the improvement is due to the effect of the foot splint alone. To date, no study has assessed whether the resting foot splint is really effective.
The aim of our current study was to determine if wearing a night splint was effective in preventing ankle plantarflexion contracture in the subacute stages after a stroke. However, the principal finding of this trial was that using a resting foot splint confers no benefit when added to personally programmed ankle stretching and exercises. Over the 3-week trial, the splint and control groups showed no statistically significant or clinically relevant differences in foot edema, passive dorsiflexion angle and FMS. These results were consistent with previous reports that compared long-duration stretching to no stretching after spinal cord injury [2223], or the use of a night splint versus a tilt table [21]. Furthermore, there were no differences between groups for foot edema, passive dorsiflexion angle, and FMS. The FAC showed a greater increase in the control than in the resting foot splint group.
One explanation for this result could be that the passive stretching applied by a resting foot splint was insufficient to produce stress on joint [2425] or long-term tissue adaptations [26] after immobilization. In addition, although instructions on proper use of the resting foot splint were given to the patients or their caregivers, it is possible that the splint was worn incorrectly. Another possible explanation is that, even though other systematic reviews concluded that regular stretching does not produce clinically important changes in ankle joint mobility, a conventional hospital rehabilitation program may be largely sufficient to prevent contractures. All included patients in our present analyses were admitted to the hospital and attended personalized rehabilitation programs. Those programs consisted not only of many repetitions of standing and active performing of everyday tasks, but also passive movements, stretching, electrical stimulation and gait training. Such treatments might have prevented ankle contracture, without the need for an adjunctive ankle splint. All other treatments should be limited, in order to evaluate the effectiveness of resting foot splints in preventing ankle contracture, but this approach would likely pose ethical problems. The third explanation for our results was that the increased muscle tone in study group might have influenced the ankle dorsiflexion angle of patients. After 3 weeks of intervention, the spasticity of the splint group was greater than the spasticity of control group (splint group, 1.19; control group, 0.65; p=0.04). Even though we failed to show a significant difference in ankle dorsiflexion improvement between groups after adjusting for initial value and spasticity after 3 weeks, it is possible that the spasticity masked the effect of splint induced tension on ankle.
Our study had several limitations. First, the sample size was small, though comparable to most other studies on contracture prevention in patients with neurologic conditions; however, larger studies are required to reveal subtle differences between groups. Second, many studies in the literature document that the process of contracture is acute [9101112131419], hence, even though we recruited patients without ankle contracture, it is possible that ongoing microscopic changes in the muscular and articular tissue blocked the resting foot splint's function in study group. In a series of studies on mouse soleus muscles, McLachlan [91011] and McLachlan and Chua [12] reported that after only 24 hour unloading, there was already a 60% shortening of muscle fiber length and sarcomere disorganization. Because we defined contracture as a dorsiflexion of the affected ankle of <10° compared to the intact ankle, baseline ankle dorsiflexion angle of both groups was below the normal range. These features might reflect already started acute process of contractures. Third, the compliance in wearing the night splint was not documented.
In summary, wearing a resting foot splint for 3 weeks did not show effects on joint mobility in a series of patients with subacute brain injury who regularly attended personalized rehabilitation program. However, this study was limited by different development of spasticity between groups and the possible early development of contracture before 3 weeks of intervention. Future research that is well controlled in development of spasticity between groups is required to clarify the efficacy of resting foot splint in the acute stage of brain injury.
References
1. Harvey LA, Herbert RD, Glinsky J, Moseley AM, Bowden J. Effects of 6 months of regular passive movements on ankle joint mobility in people with spinal cord injury: a randomized controlled trial. Spinal Cord. 2009; 47:62–66. PMID: 18574489.

2. Braddom RL. Physical medicine and rehabilitation. 4th ed. Philadelphia: Elsevier;2011. p. 1148–1149.
3. Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology. 2013; 80(3 Suppl 2):S13–S19. PMID: 23319481.

4. Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve. 2005; 31:552–571. PMID: 15714511.

5. Viosca E, Martinez JL, Almagro PL, Gracia A, Gonzalez C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch Phys Med Rehabil. 2005; 86:1234–1238. PMID: 15954065.

6. Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993; 73:447–454. PMID: 8316578.

7. Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005; 43:577–586. PMID: 15838527.

8. Botte MJ, Nickel VL, Akeson WH. Spasticity and contracture: physiologic aspects of formation. Clin Orthop Relat Res. 1988; (233):7–18. PMID: 3042237.

9. McLachlan EM. Rapid atrophy of mouse soleus muscles after tenotomy depends on an intact innervation. Neurosci Lett. 1981; 25:269–274. PMID: 7290527.

10. McLachlan EM. Modification of the atrophic effects of tenotomy on mouse soleus muscles by various hind limb nerve lesions and different levels of voluntary motor activity. Exp Neurol. 1983; 81:669–682. PMID: 6884476.

11. McLachlan EM. Atrophic effects of proximal tendon transection with and without denervation on mouse soleus muscles. Exp Neurol. 1983; 81:651–668. PMID: 6884475.

12. McLachlan EM, Chua M. Rapid adjustment of sarcomere length in tenotomized muscles depends on an intact innervation. Neurosci Lett. 1983; 35:127–133. PMID: 6856191.

13. Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol Respir Environ Exerc Physiol. 1979; 47:974–977. PMID: 511723.

14. Baker JH, Hall-Craggs EC. Changes in sarcomere length following tenotomy in the rat. Muscle Nerve. 1980; 3:413–416. PMID: 6448348.

15. Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve. 2005; 31:535–551. PMID: 15714510.

16. Sackley C, Brittle N, Patel S, Ellins J, Scott M, Wright C, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke. 2008; 39:3329–3334. PMID: 18787199.

17. Harvey LA, Herbert RD. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord. 2002; 40:1–9. PMID: 11821963.

18. Dudek N, Trudel G. Joint contractures. In : Frontera WR, Rizzo TD, Silver JK, editors. Essentials of physical medicine and rehabilitation: musculoskeletal disorders, pain, and rehabilitation. 2nd ed. Philadelphia: Elsevier;2008. p. 651–655.
19. Booth FW. Effect of limb immobilization on skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982; 52:1113–1118. PMID: 7047468.

20. Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev. 2010; (9):CD007455. PMID: 20824861.

21. Robinson W, Smith R, Aung O, Ada L. No difference between wearing a night splint and standing on a tilt table in preventing ankle contracture early after stroke: a randomised trial. Aust J Physiother. 2008; 54:33–38. PMID: 18298357.

22. Harvey LA, Batty J, Crosbie J, Poulter S, Herbert RD. A randomized trial assessing the effects of 4 weeks of daily stretching on ankle mobility in patients with spinal cord injuries. Arch Phys Med Rehabil. 2000; 81:1340–1347. PMID: 11030499.

23. Harvey LA, Byak AJ, Ostrovskaya M, Glinsky J, Katte L, Herbert RD. Randomised trial of the effects of four weeks of daily stretch on extensibility of hamstring muscles in people with spinal cord injuries. Aust J Physiother. 2003; 49:176–181. PMID: 12952517.

24. Trudel G, Uhthoff HK, Brown M. Extent and direction of joint motion limitation after prolonged immobility: an experimental study in the rat. Arch Phys Med Rehabil. 1999; 80:1542–1547. PMID: 10597804.

25. Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop Relat Res. 1987; (219):28–37. PMID: 3581580.

26. Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972; 224:231–244. PMID: 5039983.

Table 2
Clinical outcomes of intra-group and between-group differences at 3 weeks
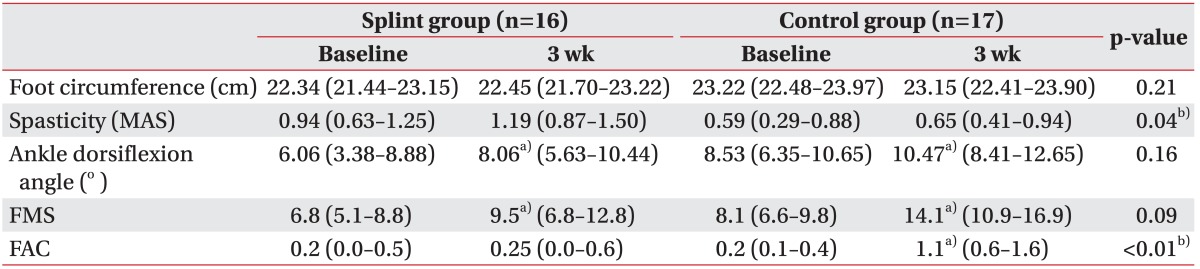
Values are presented as the mean (95% confidence interval).
MAS, Modified Ashworth Scale; FMS, Fugl-Meyer score for lower extremity; FAC, Functional Ambulation Classification.
a)p<0.05 in a Wilcoxon signed-ranks test between baseline and at 3 week.
b)p<0.05 in a Mann-Whitney U test between groups at 3 week.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download