This article has been
cited by other articles in ScienceCentral.
Abstract
Heterotopic ossification (HO) is frequently seen on rehabilitation units after spinal cord injuries, fractures, brain injuries, and limb amputations. Currently, there is no effective treatment for HO other than prophylaxis with anti-inflammatory medications, irradiation, and bisphosphonate administration. These prophylactic treatments are not effective for managing ectopic bone once it has formed. Here we describe three cases of established neurogenic HO treated with radiation therapy (RT). All patients had decreased serum alkaline phosphatase (ALP) and bone-specific ALP levels with decreased pain but increased range of motion immediately after RT. Post-treatment X-rays revealed no further growth of the HO. All patients maintained clinical and laboratory improvements 4 or 6 months after the RT. Our results suggest that RT is safe and effective in decreasing pain and activity of neurogenic HO.
Go to :

Keywords: Brain injuries, Heterotopic ossification, Radiotherapy
INTRODUCTION
Heterotopic ossification (HO) is mature lamellar bone formed in soft tissue. HO has been documented in patients with spinal cord injuries, burns, fractures, brain injury, and limb amputations [
1].
Several laboratory tests have been used to determine whether their ability in predicting or identifying the formation of HO, including serum alkaline phosphatase (ALP) levels [
2]. Bone-specific alkaline phosphatase (BALP) level has been proven to be a sensitive and reliable indicator of bone metabolism [
3]. When HO is accompanied by joint pain and ankylosis, it can limit rehabilitation. Therefore, it is important to treat HO. Radiation therapy (RT) has been found to have prophylactic effect against HO [
4]. However, only a few reports are available on its short-term therapeutic effects in established HO [
56]. Here, we report the results of 4–6 month follow-up evaluation for three patients with HO after brain injury who have undergone RT. For the first time, we serially evaluated the changes in the range of motion (ROM) of the involved joints, pain severity, and serum bone formation markers (BALP) after RT.
Go to :

CASE REPORTS
Case 1
A 23-year-old man with a diffuse axonal brain injury and multiple fractures caused by a fall 1 year earlier was admitted to Department of Rehabilitation Medicine, Eulji Hospital. Brain magnetic resonance imaging (MRI) results are shown in
Fig. 1. He had undergone open reduction and internal fixation for left distal tibia and fibula, right calcaneus, and right proximal tibia fractures in the Department of Orthopedic Surgery. After the injury, he suffered from bilateral lower extremity weakness with moderate cognitive impairment (Korean-Mini Mental State Examination score=22). HO was diagnosed at the right hip and both knee joints at 6 months after the accident (
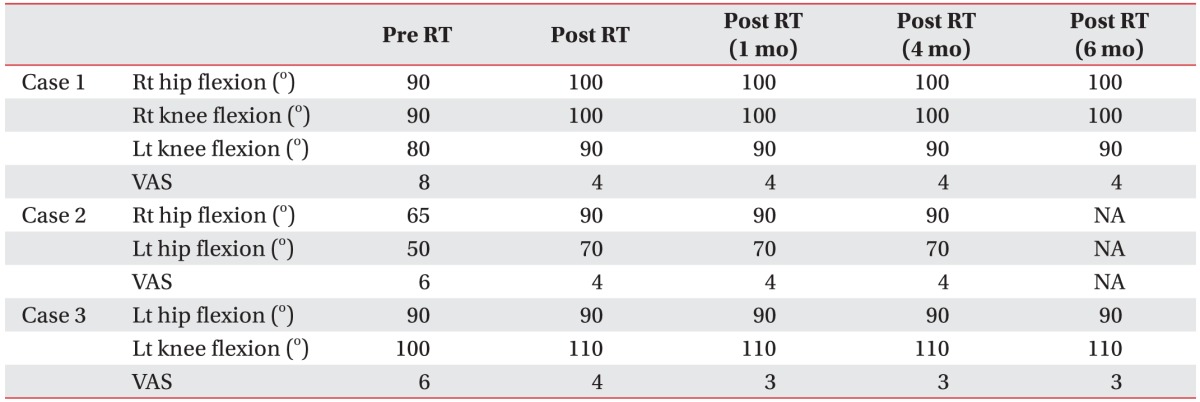
Fig. 2). At this point, his serum ALP level and BALP level were 119 IU/L (normal range, 80–270 IU/L) and 22.3 IU/L (normal range, 4.5–16.9 IU/L), respectively. Although he had been taking 600 mg oral etidronate disodium daily for 6 months and undergoing physical therapy, the passive ROM of the involved joints was limited. He complained of pain aggravated by ROM exercise (visual analog scale [VAS] score=8). One year after the accident, his serum ALP level and BALP level were progressively elevated to 213 IU/L and 67.6 IU/L, respectively. To relieve the pain, the patient underwent 10 days of RT for a total radiation dose of 20 Gy in 2 Gy fractions on the right hip and both knee, respectively. After completing the RT, he had mild improvement in joint ROM with decreased pain on exercise (
Table 1). He had no side effect such as bone marrow suppression. His serum ALP and BALP levels were decreased immediately after the RT to 172 IU/L and 48.6 IU/L, respectively (
Fig. 3). Six months after the RT, he maintained both clinical and laboratory improvements (
Table 1,
Fig. 3).
 | Fig. 1(A) Gradient recalled echo brain magnetic resonance imaging (MRI) in case 1 showing a dark signal intensity at the gray/white matter interfaces in the bilateral superior frontal gyri suggestive of diffuse axonal injury. In case 2, brain MRI showing an enhancing mass at the falx (B) and in the parietal area with a cerebrospinal fluid cleft suggestive of extra-axial meningioma (C). (D) In case 3, brain computed tomography showing an intracerebral hemorrhage in the right basal ganglia and an intraventricular hemorrhage.
|
 | Fig. 2In case 1, AP radiographs of the right hip (A) and both knees showing HO (B). Whole body bone scan showing increased uptake at the right hip and both knees (C). (D) In case 2, AP radiographs of both hips showing HO. In case 3, AP radiograph of the left hip (E) and lateral radiograph of the left knee showing HO (F). Whole body bone scan showing increased uptake at the left hip and knee (G). AP, anteroposterior; HO, heterotopic ossification.
|
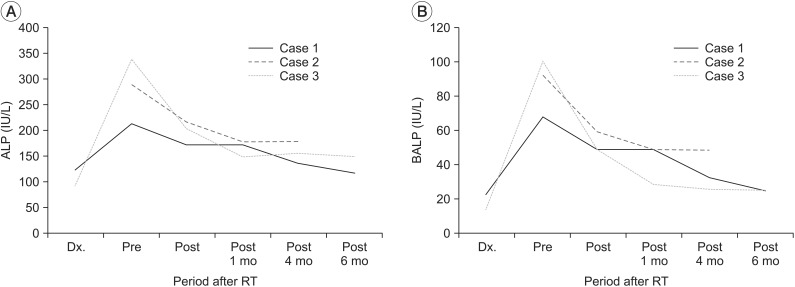 | Fig. 3(A) Serum alkaline phosphatase (ALP) and (B) bone-specific alkaline phosphatase (BALP) levels are dramatically decreased immediately after radiation therapy (RT) in all three cases. These values remained low or decreased further throughout the follow-up period. Dx, the time of the initial diagnosis of heterotopic ossification.
|
Table 1
Changes in VAS scores and passive range of motion in the three patients after RT

Case 2
A 52-year-old man who had been diagnosed with a meningioma 12 years earlier was admitted to Department of Rehabilitation Medicine, Eulji Hospital. Brain MRI results are shown in
Fig. 1. He had undergone craniotomies and tumor removal at the Department of Neurosurgery twice (12 and 9 years earlier). He had progressive weakness of all four extremities with loss of ambulation. He was diagnosed with HO in both hip joints (
Fig. 2) approximately at 3 months before admission to our rehabilitation department. Although the patient had been taking 1,200 mg of oral etidronate disodium daily and undergone physical therapy, his passive ROM was limited and he complained of pain (VAS=6). His serum ALP and BALP levels were elevated to 289 IU/L and 92.1 IU/L, respectively, at the time of admission. The patient was given 10 days of RT to relieve pain in a total radiation dose of 20 Gy with 2 Gy per fraction on both hips, respectively. After RT, his ROM was improved and his pain was decreased (
Table 1). His serum ALP and BALP levels were decreased immediately after RT to 216 IU/L and 58.7 IU/L, respectively (
Fig. 3). After RT, he developed leukocytopenia (3,130/µL), which had normalized by 1 month after RT. After 4 months, his improvement was maintained (
Table 1,
Fig. 3). At that time, he was diagnosed with recurrent meningioma. Therefore, he underwent reoperation.
Case 3
A 35-year-old man with a spontaneous right intracranial/intraventricular hemorrhage (
Fig. 1) 6 months earlier was admitted to Department of Rehabilitation Medicine, Eulji Hospital. At the time of injury, he had undergone burr hole trephination and closed drainage in the Department of Neurosurgery. He suffered from left hemiplegia. HO was developed at the left hip and knee joints (
Fig. 2) at 3 months after the stroke. Although he had been taking oral etidronate disodium daily and receiving physical therapy, he developed contractures and complained of pain on exercise (VAS=6). Five months after the stroke, his serum ALP and BALP levels were progressively elevated to 340 IU/L and 101 IU/L, respectively. At that point, to relieve pain, the patient was given 10 days of RT for a total radiation dose of 20 Gy in 2 Gy fractions left hip and knee, respectively. Immediately after completing the RT, he only had mild improvement of the ROM in the left knee joint with decreased pain (
Table 1). He had no side effect such as bone marrow suppression. Immediately after RT, his serum ALP and BALP levels were decreased to 203 IU/L and 47.9 IU/L, respectively. Six months later, his clinical and laboratory improvements were maintained (
Table 1,
Fig. 3).
Go to :

DISCUSSION
Neurogenic HO has been reported in 11%–76% of patients with traumatic brain injury, of which 10%–20% of cases are clinically significant [
7]. To decrease further complications after HO, early diagnosis and intervention are crucial [
8].
All our three patients complained of spontaneous pain or pain aggravated by physical therapy of the involved joint. In addition, their BALP levels were elevated in all cases, reflecting the increase of bone-formation activity of HO. Pain is common with HO and it is associated with increased HO activity [
9]. RT can effectively block the earliest steps after HO by arresting osteoblast differentiation from pluripotent mesenchymal cells [
10]. In addition, RT may decrease the pain associated with inflammatory reaction around the site of HO. It might be involved in the ablation of pain receptors [
5]. In this case series, RT was conducted at the course of their clinical and laboratory deterioration (ALP, BALP). All three patients showed clinical and laboratory improvement immediately after RT (
Table 1,
Fig. 3). Therefore, the improvement can be interpreted as the effect of RT rather than natural progress or other influences.
Few reports are available on the effects of RT in established neurogenic HO. We used the same RT protocol that Schaeffer and Sosner [
4] used to treat two cases of established HO with pain and limited ROM of the involved hips and right elbow. In those cases, immediately after the RT, the pain was decreased, the ROM was increased, and the serum ALP level was decreased. Jang et al. [
6] have also reported that RT can decrease pain and serum ALP level while increasing the ROM of treated joints in a case of established HO. Their patient showed pancytopenia after the RT but recovered to within normal range at 1 month after RT [
6]. Similarly, in one of our cases (case 2), his serum white blood cell count was decreased after RT but had normalized at 1 month after RT.
In our case series, the ROM of the involved joints was improved somewhat and the pain severity was decreased after RT. Such improvements were maintained during the 6-month follow-up period. Their serum ALP and BALP levels were also decreased dramatically immediately after RT in all three cases. Such decrease was maintained throughout the follow-up period. Post-treatment X-rays revealed no further growth of the HO. Our results suggest that RT can be used as safe and effective method to immediately decrease pain and activity of neurogenic established HO. Such effect can maintain for 4–6 months after RT.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download