1. Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997; 20:1477–1486. PMID:
9390659.

2. Werner RA, Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011; 44:597–607. PMID:
21922474.

3. Al-Shekhlee A, Shapiro BE, Preston DC. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve. 2003; 27:517–526. PMID:
12707972.

4. Rubin DI. Technical issues and potential complications of nerve conduction studies and needle electromyography. Neurol Clin. 2012; 30:685–710. PMID:
22361380.

5. Buchberger W, Judmaier W, Birbamer G, Lener M, Schmidauer C. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992; 159:793–798. PMID:
1529845.

6. Koenig RW, Pedro MT, Heinen CP, Schmidt T, Richter HP, Antoniadis G, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009; 26:E13.

7. Gellhorn AC, Carlson MJ. Inter-rater, intra-rater, and inter-machine reliability of quantitative ultrasound measurements of the patellar tendon. Ultrasound Med Biol. 2013; 39:791–796. PMID:
23465140.

8. Reimers CD, Schlotter B, Eicke BM, Witt TN. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. J Neurol Sci. 1996; 143:46–56. PMID:
8981297.

9. Schmidt R, Voit T. Ultrasound measurement of quadriceps muscle in the first year of life: normal values and application to spinal muscular atrophy. Neuropediatrics. 1993; 24:36–42. PMID:
8474609.

10. Kamala D, Suresh S, Githa K. Real-time ultrasonography in neuromuscular problems in children. J Clin Ultrasound. 1985; 13:465–468. PMID:
3932477.
11. Zuberi SM, Matta N, Nawaz S, Stephenson JB, McWilliam RC, Hollman A. Muscle ultrasound in the assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord. 1999; 9:203–207. PMID:
10399745.

12. Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009; 35:443–446. PMID:
19081667.

13. Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010; 41:32–41. PMID:
19722256.

14. Maurits NM, Bollen AE, Windhausen A, De Jager AE, Van Der Hoeven JH. Muscle ultrasound analysis: normal values and differentiation between myopathies and neuropathies. Ultrasound Med Biol. 2003; 29:215–225. PMID:
12659909.

15. Bargfrede M, Schwennicke A, Tumani H, Reimers CD. Quantitative ultrasonography in focal neuropathies as compared to clinical and EMG findings. Eur J Ultrasound. 1999; 10:21–29. PMID:
10502636.

16. Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE. Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med. 1993; 12:73–77. PMID:
8468739.

17. Arts IM, van Rooij FG, Overeem S, Pillen S, Janssen HM, Schelhaas HJ, et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008; 34:354–361. PMID:
17964067.

18. de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol. 1992; 45:373–376. PMID:
1569433.

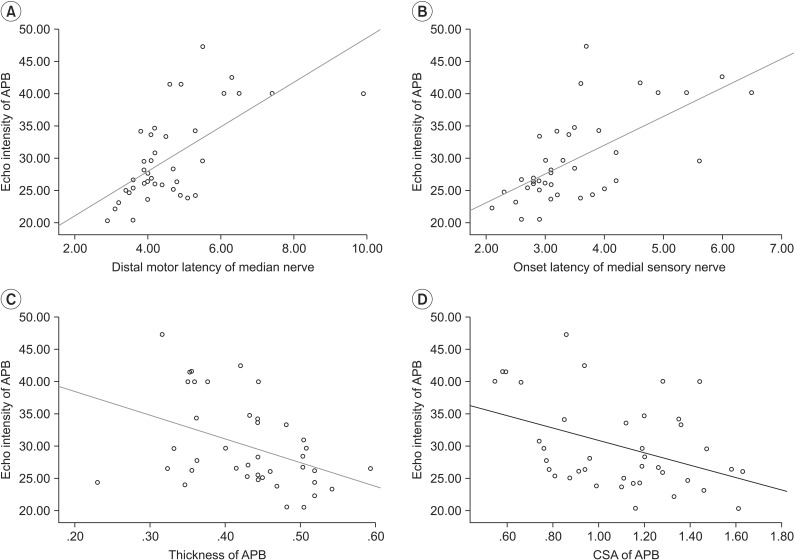
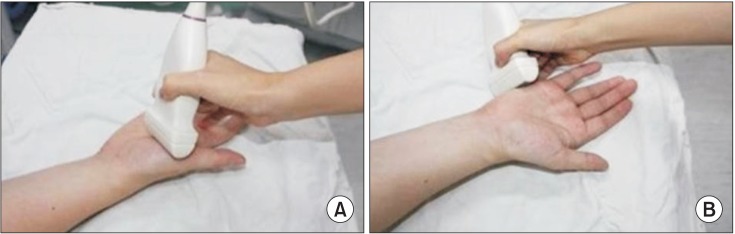
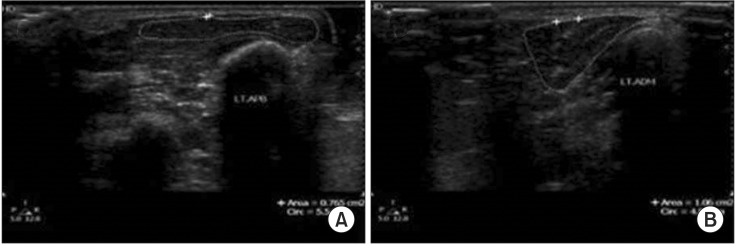
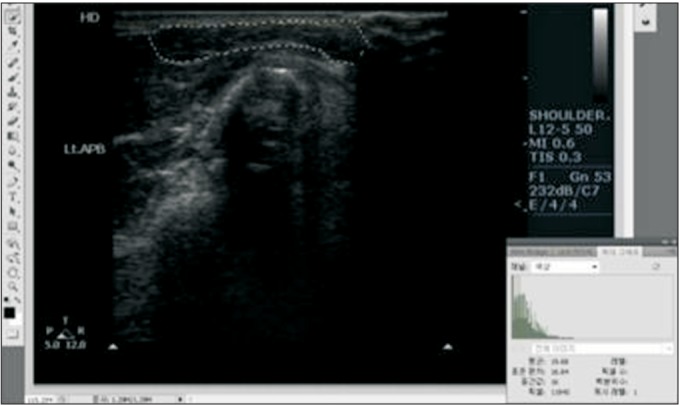
19. Kim JS, Seok HY, Kim BJ. The significance of muscle echo intensity on ultrasound for focal neuropathy: the median- to ulnar-innervated muscle echo intensity ratio in carpal tunnel syndrome. Clin Neurophysiol. 2016; 127:880–885. PMID:
25998202.

20. Scholten RR, Pillen S, Verrips A, Zwarts MJ. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve. 2003; 27:693–698. PMID:
12766980.

21. Dumitru D, Amato AA, Zwarts M. Electrodiagnostic medicine. 2nd ed. Philadelphia: Hanley & Belfus;2002. p. 1062.
22. Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997; 96:211–217. PMID:
9325471.

23. Fleiss JL. Design and analysis of clinical experiments. New York: John Wiley & Sons;1999. p. 1–28.
24. Schmidt WA, Schmidt H, Schicke B, Gromnica-Ihle E. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis. 2004; 63:988–994. PMID:
15249327.

25. Beekman R, Visser LH. Sonography in the diagnosis of carpal tunnel syndrome: a critical review of the literature. Muscle Nerve. 2003; 27:26–33. PMID:
12508291.

26. Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008; 37:679–693. PMID:
18506712.

27. Simon NG, Ralph JW, Lomen-Hoerth C, Poncelet AN, Vucic S, Kiernan MC, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015; 52:221–230. PMID:
25388871.

28. Wallwork TL, Hides JA, Stanton WR. Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. J Orthop Sports Phys Ther. 2007; 37:608–612. PMID:
17970407.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download