INTRODUCTION
MATERIALS AND METHODS
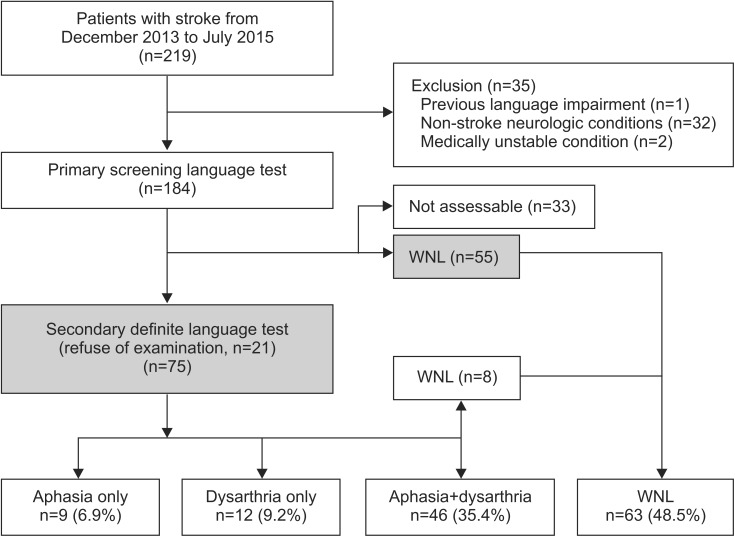
Subjects
Language and speech evaluations
Functional evaluations
Statistical analysis
RESULTS
Demographic characteristics of recruited post-stroke patients
Table 1
Demographic characteristics of recruited post-stroke patients (n=130)
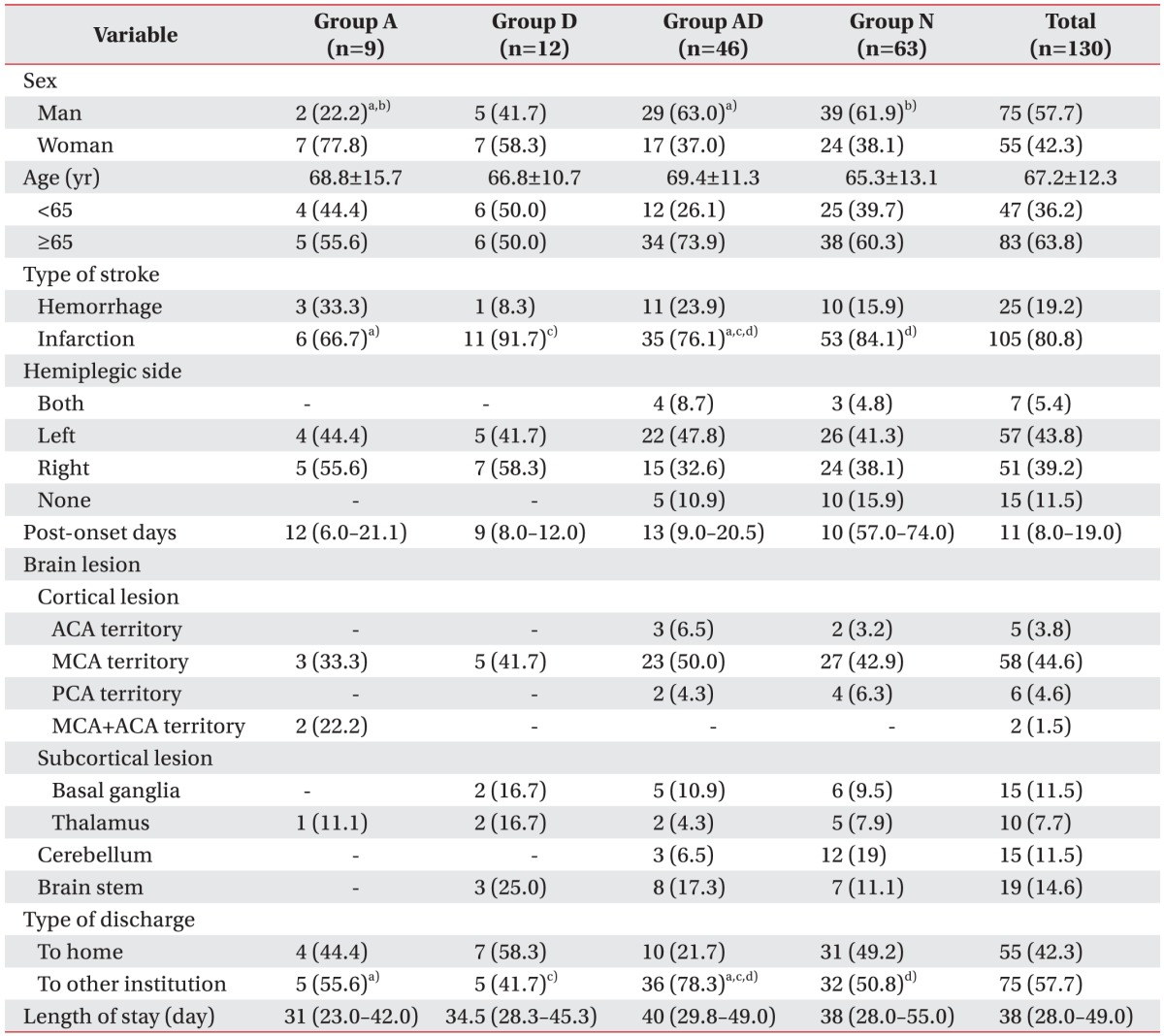
Values are presented as number (%) or mean±standard deviation or median (interquartile range).
Statistically significant differences a)between group A and group AD, b)between group A and group N, c)between group D and group AD, and d)between group AD and group N by a χ2 test between paired groups (p<0.05).
Comparison of initial and discharge NIHSS, MMSE-K, EQ-5D-3L, K-MBI, and MI scores by study group
Table 2
Comparison of initial and discharge NIHSS, MMSE-K, EQ-5D-3L, K-MBI, and MI scores by study group
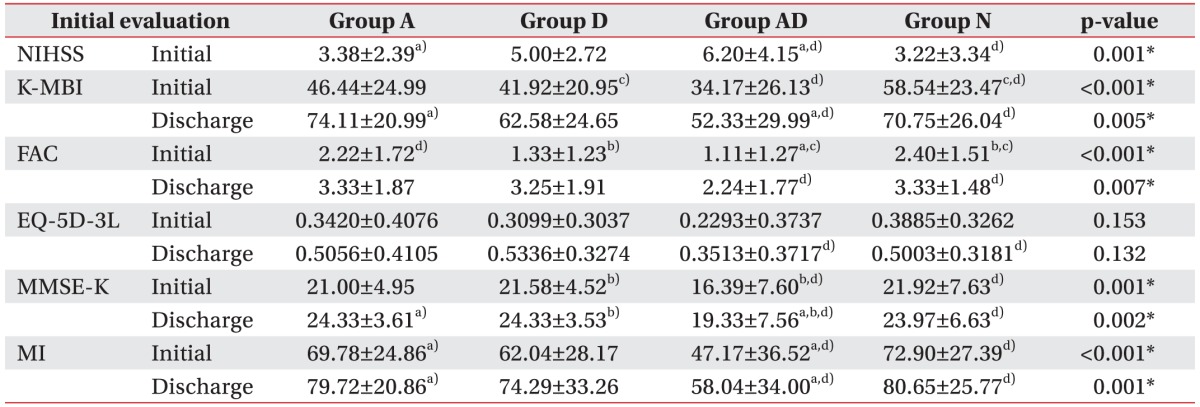
Values are presented as mean±standard deviation.
K-NIHSS, Korean version of National Institutes of Health Stroke Scale; FAC, Functional Ambulation Category; MMSE-K, Korean version of Mini-Mental State Examination; EQ-5D-3L, European Quality of Life-5 Dimensions 3 Level; K-MBI, Korean version of Modified Barthel Index; MI, Motricity Index of hemiplegic side.
*p<0.05, statistically significant differences a)between group A and group AD, b)between group D and group AD, c)between group D and group N, and d)between group AD and group N based on one-way ANOVA test with LSD multiple comparison.
Comparison of K-MBI, FAC, EQ-5D-3L, MMSE-K, and MI scores at the initial and discharge times
Table 3
Comparison between initial and discharge K-MBI, FAC, EQ-5D-3L, MMSE-K, and MI scores
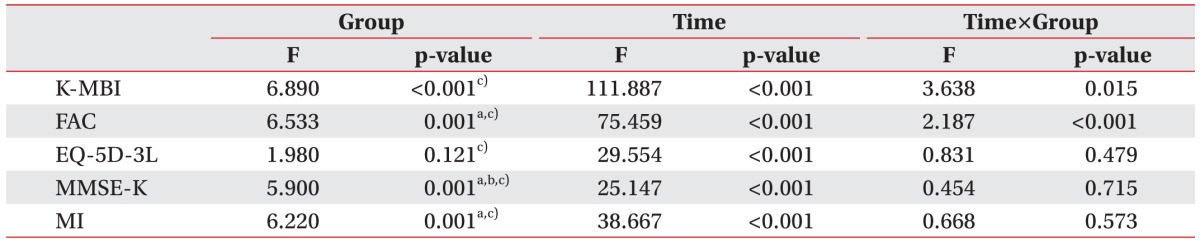
MMSE-K, Korean version of Mini-Mental State Examination; FAC, Functional Ambulation Category; EQ-5D-3L, European Quality of Life-5 Dimensions 3 Level; K-MBI, Korean version of Modified Barthel Index; MI, Motricity Index of hemiplegic side.
Statistically significant differences a)between group A and group AD, b)between group D and group AD, and c)between group AD and group N based on repeated measures ANOVA test with LSD multiple comparison (p<0.05).
Result of multivariable linear regression analysis to show the functional improvements regarding the initial severity and age
Table 4
Result of multivariable linear regression analysis to show functional improvements regarding the initial severity and age
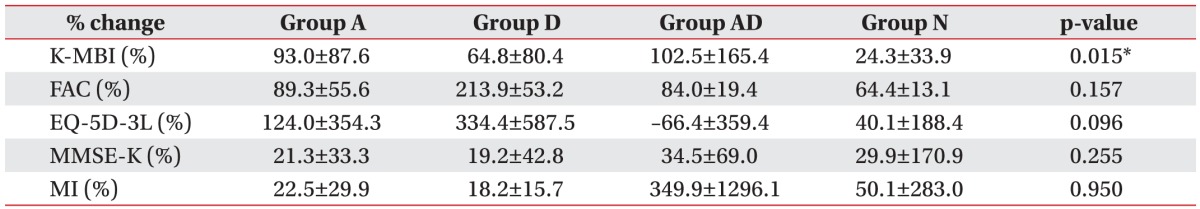
Values are presented as mean±standard deviation.
K-MBI, Korean version of Modified Barthel Index; FAC, Functional Ambulation Category; EQ-5D-3L, European Quality of Life-5 Dimensions 3 Level; MMSE-K, Korean version of Mini-Mental State Examination; MI, Motricity Index of hemiplegic side.
*p<0.05, multivariable linear regression analysis was performed with adjustment by each initial NIHSS score and age.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download