Abstract
Objective
To investigate the association of family history of stroke with functional outcomes in stroke patients in Korea.
Methods
A case-control study was conducted. A total of 170 patients who were admitted to a rehabilitation unit were included. Risk factors for stroke such as age, sex, diabetes mellitus, hypertension, atrial fibrillation, smoking, high blood cholesterol and homocysteine level, obesity, and family history of stroke were taken into account. Stroke subtypes were the following: large vessel infarct, small vessel infarct, embolic infarct, subarachnoid hemorrhage, and intracranial hemorrhage. Stroke severity as assessed with the National Institutes of Health Stroke Scale (NIHSS), functional outcomes using the Korean version of the Modified Barthel index (K-MBI), Functional Independence Measurement (FIM), and cognitive function using the Korean version of Mini-Mental State Examination (K-MMSE) were assessed at admission and discharge.
Go to : 
Stroke is one of the major causes of impairment and mortality in developed countries. Rates of stroke survival with disability are variable worldwide, but several studies have reported predictors of post-stroke functioning to be patient age, stroke subtype, severity, location and size of stroke, and family history of stroke. Family history is a vital piece of medical information because family members partially share genes, environment, and lifestyles, which play significant roles in the development of stroke [12]. Also, knowledge of family history is an excellent starting point in investigating genetic factors in stroke, their effects on stroke risk, and prognoses.
Previous studies on family history usually focused on its role as a risk factor rather than as a prognostic factor [23456]. There has been no consensus as to whether family history is a prognostic factor [23456]. This is partially because other risk factors for stroke, such as diabetes mellitus, hypertension, and hyperhomocysteinemia, also have familial connections, so the effects of each are difficult to tease apart.
As family history is closely related to genetics, individuals of different ethnicities may have different risk factors and prognoses. In fact, slight discrepancies have been previously identified between races [46789].
One study reported that a family history of stroke was related to ischemic stroke subtype and to poor functional status, as assessed using the modified Rankin Scale, at discharge [7], while no significant relationship to outcome was reported in another study [9].
To our knowledge, studies on the association of family history of stroke and functional outcomes in Asian populations are scarce. One article from China reports only an indirect association between family history and metabolic syndrome, and discusses the effects of metabolic syndrome on stroke [10].
The aim of this study was to obtain preliminary data on the relation of family history of stroke with functional outcomes and stroke severity in stroke patients in Korea.
Go to : 
A retrospective case-control study was performed. Electronic medical records of stroke patients who were admitted to the rehabilitation unit of Dankook Universitiy Hospital from May 2008 to June 2013 were screened. Microsoft SQL Server 2014 Express (Microsoft, Redmond, WA, USA) was used to extract data from the hospital's electronic medical record database.
A total of 184 patients were diagnosed with stroke during the period in question, but 14 were excluded because rehabilitative treatments were discontinued due to other medical conditions. Thus, data from 170 patients was analyzed. Participants were divided into two groups: those with a family history of stroke, and those with no family history of stroke-FHx(+) vs. FHx(-), respectively.
Demographic characteristics such as age and gender were recorded. Patients or their family members were asked whether any first-degree relative had experienced a stroke. A first-degree relative is a family member who shares a significant amount of genetic material with a particular individual. First-degree relatives include parents, offspring, and siblings [11].
Risk factors for stroke including hypertension, diabetes mellitus, atrial fibrillation, and hypercholesterolemia were also recorded at the time of admission. Hypertension was identified based on a previous diagnosis or current treatment with antihypertensive drugs. Atrial fibrillation was evaluated with electrocardiography upon admission. Hypercholesterolemia was diagnosed when serum cholesterol levels exceeded 250 mg/dL, and hyperhomocysteinemia when serum levels were greater than 15.39 µmol/L. A poorly controlled glucose level was defined as serum hemoglobin A1c (HbA1c) >6.5%. Body mass index was calculated using measurements of body weight and height taken at the time of admission. A patient was marked positive for smoking if he/she had been smoking for more than 5 years. The total length of stay in rehabilitation (in days) was recorded as a measure of treatment intensity. Stroke types were classified into 5 categories using the Harvard Cooperative Stroke Registry: large vessel infarct, small vessel infarct, embolic infarct, subarachnoid hemorrhage (SAH), and intracranial hemorrhage (ICH) [12].
Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) score at admission and discharge [13].
Functional outcomes were assessed at admission and discharge using the Korean version of the Modified Barthel Index (K-MBI) and the Functional Independence Measure (FIM) [1415]. Cognitive functions were evaluated using the Korean version of Mini-Mental State Examination (K-MMSE) at admission and discharge [16].
All statistical analyses were performed using STATA/MP 13.0 for Windows (StataCorp LP, College Station, TX, USA). Baseline characteristics and outcome measures between the two groups (with or without family history of stroke), were compared by a chi-square test using categorical variables, and by the Mann-Whitney U test using continuous variables. Changes between status at admission and discharge were analyzed using the Wilcoxon signed-rank test and Mann-Whitney U test. Normality was measured with the Shapiro-Wilk W test. Statistical significance was set at p-value <0.05. Data are given as mean±standard deviation.
Go to : 
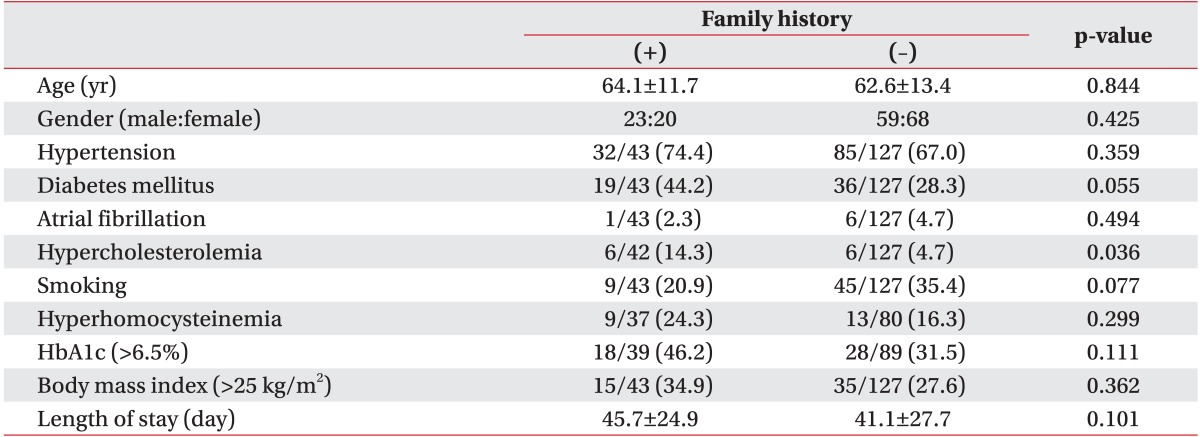
A total of 170 subjects (82 men and 88 women, aged 63.0±13.0 years) were included in this study. Forty-three patients (25.3%) reported that at least one first-degree relative had experienced a stroke (FHx(+) group). The FHx(+) and FHx(-) groups did not show significant differences in age, gender, known risk factors for stroke, total length of stay in rehabilitation; there was a significant difference in hypercholesterolemia (Table 1) such that there was a significantly higher incidence of hypercholesterolemia in the FHx(+) group (Table 1).
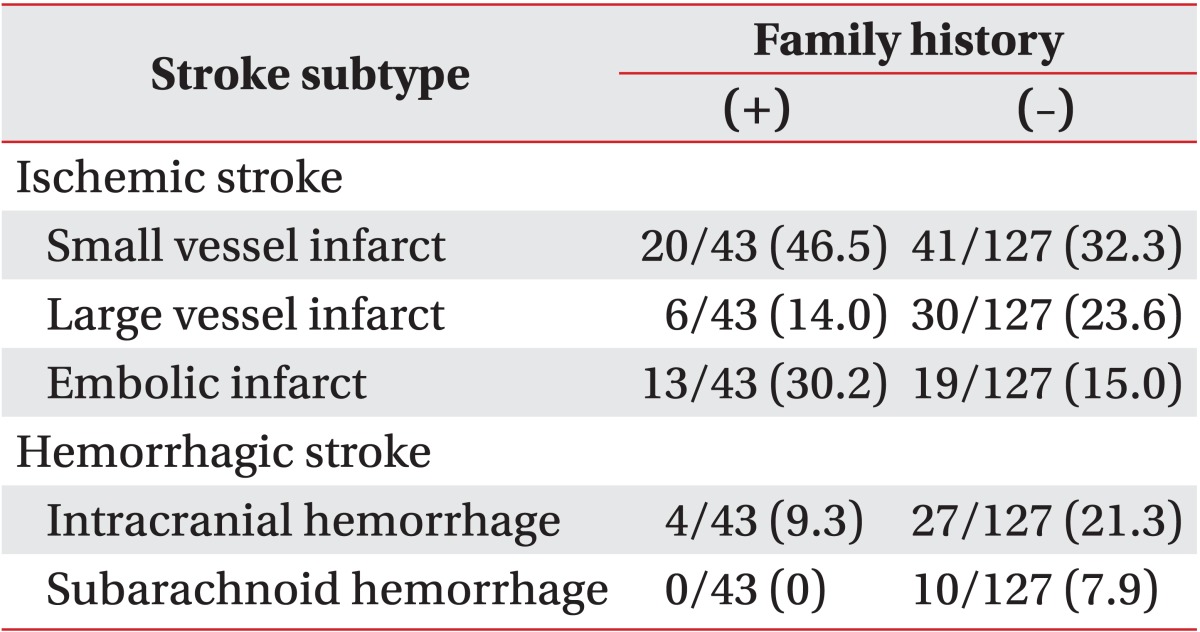
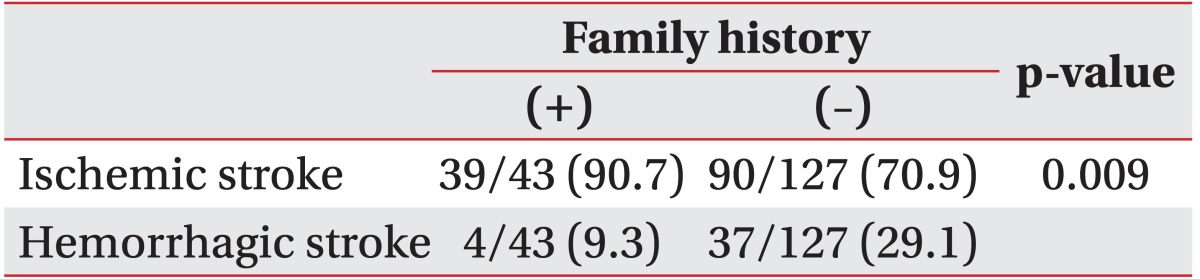
The distributions of stroke subtypes were significantly different between the groups (p=0.011 by chi-square test). Stroke subtypes in the FHx(+) group were 46.5% small vessel infarct, 30.2% embolic infarct, 14.0% large vessel infarct, and 9.3% intracranial hemorrhage. No individual in the FHx(+) group had a subarachnoid hemorrhage (Table 2). Stroke subtypes in the FHx(-) group were 32.3% small vessel infarct, 23.6% large vessel infarct, 21.3% intracranial hemorrhage, 15.0% multiple embolic infarct, and 7.9% subarachnoid hemorrhage (Table 2). When classified into ischemic vs. hemorrhagic stroke, ischemic stroke was more common in the FHx(+) group than in the FHx(-) group (90.7% vs. 70.9%, respectively; p=0.009 by chi-square test) (Table 3).
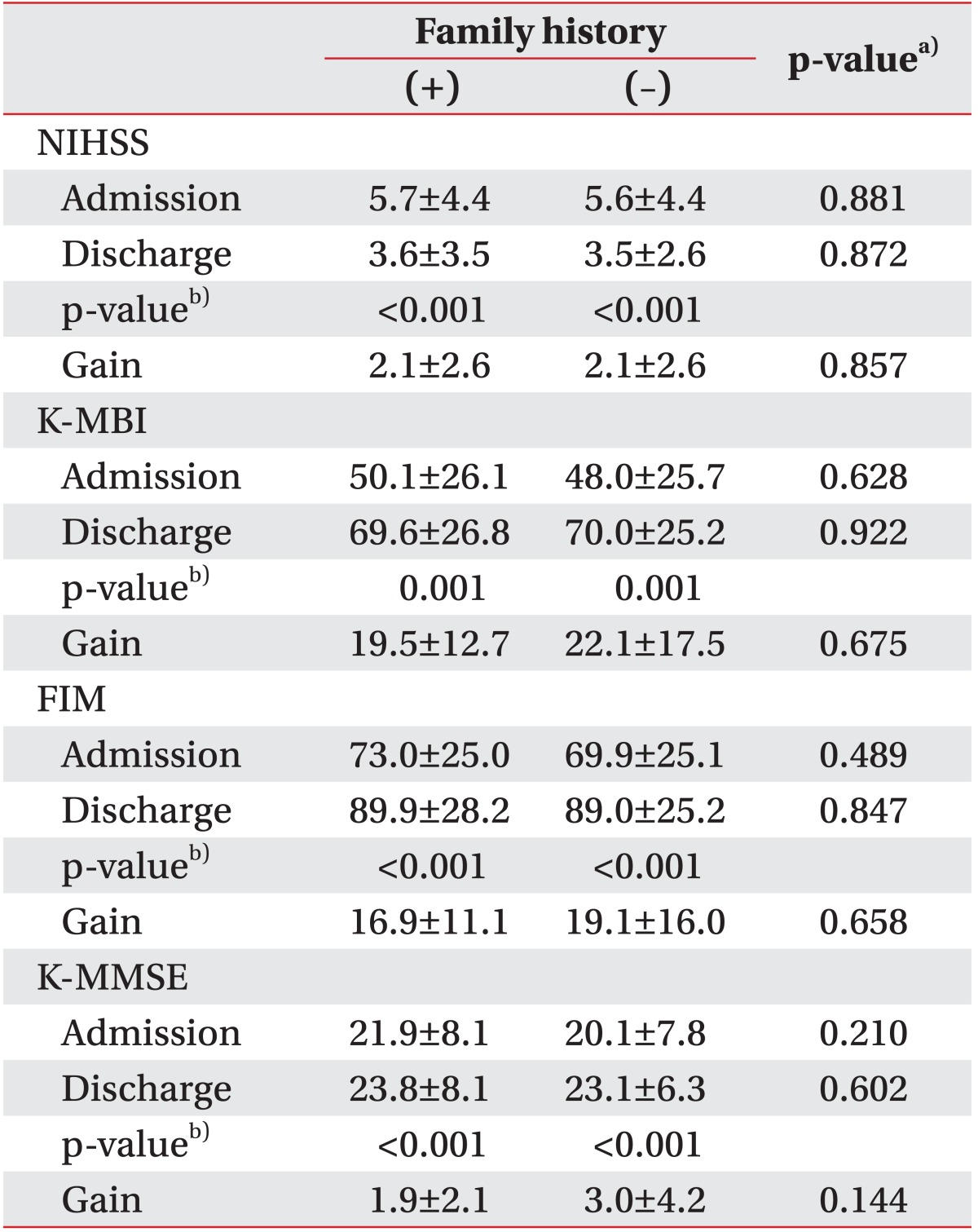
The K-MBI, FIM, K-MMSE, and NIHSS scores were not significantly different between the two groups at admission or discharge. There were significant changes in all outcome variables between admission and discharge, representing functional improvements and neurologic recovery (Table 4). Family history of stroke, FHx(+) or FHx(-), did not have a significant effect on the gains in the NIHSS, K-MBI, FIM, and K-MMSE scores (Table 4).
Even when stroke outcome was divided into favorable (K-MBI ≥90) or unfavorable (K-MBI <90), a family history of stroke had no significant effect on outcomes (favorable vs. poor outcomes, 31:12 in the FHx(+) group and 87:40 in the FHx(-) group; p=0.659 by a chi-square test).
Because no subject in the FHx(+) had a SAH, it was impossible to evaluate the relationship between family history and functional outcome in SAH. Considering all other stroke subtypes, recovery, as assessed by the NIHSS, K-MBI, FIM, and K-MMSE, was not significantly different between patients with and without a family history of stroke (data not presented).
Go to : 
Our results are consistent with previous reports that showed no significant association of family history with stroke outcome [9]. This study contributes to the ongoing controversy concerning whether or not a family history of stroke can be considered a prognostic factor [79]; a family history of stroke has already been well established as a risk factor of stroke occurrence [23456]. We found that stroke subtypes differed depending on family history, as ischemic strokes were more common in individuals in the FHx(+) group. This result is also consistent with previous findings [27]. A previous study showed that a family history of stroke was associated with small vessel infarcts or large vessel infarcts. However, a family history of stroke was more closely associated with small vessel infarcts and embolic infarcts in our study. Although hypertension is attributable to environmental factors such as smoking, diet and exercise, etc., genetics also strongly influence the prevalence of hypertension [17]. This may explain why small vessel infarcts were more common in patients with a family history of stroke. A family history of atrial fibrillation has been shown to increase the risk of atrial fibrillation [18]. Genome-wide association studies have found 27 genetic variants that are associated with an increased risk of myocardial infarction [19]. Increased risks of atrial fibrillation and myocardial infarction together may affect the incidence of embolic infarcts.
In a previous study, a history of stroke in a first-degree relative was associated with poor functional outcomes (modified Rankin Scale score ≥2 at discharge) [7]. In contrast, functional outcomes, as evaluated using primarily the K-MBI and the FIM in our study, did not differ in patients with and without a family history. To eliminate measurement effects of outcome variables (e.g., dichotomous vs. continuous variables), the K-MBI scores were divided into favorable or poor functional outcomes. Still, no differences were found between the FHx(+) and FHx(-) groups.
The above inconsistencies could be caused by differences in demographics or baseline characteristics. Genetic, national, social, and environmental factors could all exert effects on functional outcomes in stroke patients. Ethnic and genetic traits specific to Koreans could contribute to the discrepancy between the previous results [7] and those presented here. There have been reports of ethnicity affecting stroke risk and type [820], so it is reasonable to think that the usefulness of family history as a prognostic factor may differ between the Korean population and other ethnic populations.
Some limitations of this study need to be discussed. The sample size was small, and was limited to one university hospital. However, distributions of gender, stroke subtypes, and ratio of positive family history of stroke were quite similar to those used in previous studies [22122]. This suggests that the characteristics of our sample did not deviate greatly from those of the population of stroke patients as a whole. Another limitation was the absence of long-term follow-ups. Nevertheless, the length of stay of 42.3±27.0 days does cover the critical period for stroke rehabilitation [23].
The sample size that would have been able to detect significant differences between favorable outcomes (favorable outcome 27.9% in FHx(+) group and 31.5% in FHx(-) group), with a test power of 80%, significance level of 0.05, and effect size of 0.5, was calculated to be 5,056 subjects. It would be impossible to obtain a sample size of this magnitude in one hospital or by a few individual researchers. Initiating a multi-center trial or enlarging the stroke rehabilitation registry would be necessary to conduct research with sufficient statistical power.
Evaluating prognostic factors very soon after admission to the rehabilitation unit could be vital in the effort to predict outcomes, manage rehabilitation programs precisely, and inform patients and family members. A large-cohort study using the data from a few stroke registries, such as the Brain Rehabilitation Registry or the Korea Stroke Registry, would be needed to fully elucidate the relationship between family history of stroke and functional outcome of stroke in Korea. Data from our study can be used to suggest the necessary sample size, and to determine feasible outcome measures.
In conclusion, a family history of stroke was significantly associated with the incidence of ischemic stroke, but not with functional outcomes in stroke patients. Other risk factors and prognostic factors were not different between patients with or without a family history of stroke.
Go to : 
References
1. Graffagnino C, Gasecki AP, Doig GS, Hachinski VC. The importance of family history in cerebrovascular disease. Stroke. 1994; 25:1599–1604. PMID: 8042210.

2. Jerrard-Dunne P, Cloud G, Hassan A, Markus HS. Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke. 2003; 34:1364–1369. PMID: 12714707.
3. Jood K, Ladenvall C, Rosengren A, Blomstrand C, Jern C. Family history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2005; 36:1383–1387. PMID: 15933254.
4. Aycock DM, Kirkendoll KD, Coleman KC, Clark PC, Albright KC, Alexandrov AW. Family history of stroke among African Americans and its association with risk factors, knowledge, perceptions, and exercise. J Cardiovasc Nurs. 2015; 30:E1–E6. PMID: 24598552.

5. Debette S, Goeggel Simonetti B, Schilling S, Martin JJ, Kloss M, Sarikaya H, et al. Familial occurrence and heritable connective tissue disorders in cervical artery dissection. Neurology. 2014; 83:2023–2031. PMID: 25355833.

6. O'Neill SM, Rubinstein WS, Wang C, Yoon PW, Acheson LS, Rothrock N, et al. Familial risk for common diseases in primary care: the Family Healthware Impact Trial. Am J Prev Med. 2009; 36:506–514. PMID: 19460658.
7. Lisabeth LD, Smith MA, Brown DL, Uchino K, Morgenstern LB. Family history and stroke outcome in a bi-ethnic, population-based stroke surveillance study. BMC Neurol. 2005; 5:20. PMID: 16262890.

8. Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004; 160:376–383. PMID: 15286023.

9. Harmsen P, Lappas G, Rosengren A, Wilhelmsen L. Long-term risk factors for stroke: twenty-eight years of follow-up of 7457 middle-aged men in Goteborg, Sweden. Stroke. 2006; 37:1663–1667. PMID: 16728686.
10. Mi D, Zhang L, Wang C, Liu L, Pu Y, Zhao X, et al. Impact of metabolic syndrome on the prognosis of ischemic stroke secondary to symptomatic intracranial atherosclerosis in Chinese patients. PLoS One. 2012; 7:e51421. PMID: 23251528.

11. Genetics Home Reference. First-degree relative [Internet]. Bethesda: Genomics Home Reference, National Library of Medicine;2015. cited 2015 Nov 10. Available from: http://ghr.nlm.nih.gov/glossary=firstdegreerelative.
12. Mohr JP, Caplan LR, Melski JW, Goldstein RJ, Duncan GW, Kistler JP, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978; 28:754–762. PMID: 567291.

13. Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale: extension to nonneurologists in the context of a clinical trial. Stroke. 1997; 28:307–310. PMID: 9040680.
14. Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, et al. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007; 31:283–297.
15. Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med. 1994; 26:115–119. PMID: 7801060.
16. Park JH. Modification of the mini-mental state examination for use in the elderly in a non-western society. Part 1: Development of Korean version of mini-mental state examination. Int J Geriatr Psychiatry. 1990; 5:381–387.

17. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001; 104:545–556. PMID: 11239411.

18. Palatini P. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004; 292:1174–1175. PMID: 15353527.

19. O'Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. 2011; 365:2098–2109. PMID: 22129254.
20. Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation. 2007; 116:2157–2164. PMID: 17967776.
21. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. PMID: 7678184.

22. Polychronopoulos P, Gioldasis G, Ellul J, Metallinos IC, Lekka NP, Paschalis C, et al. Family history of stroke in stroke types and subtypes. J Neurol Sci. 2002; 195:117–122. PMID: 11897241.

23. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009; 10:861–872. PMID: 19888284.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download