This article has been
cited by other articles in ScienceCentral.
Abstract
Japanese encephalitis (JE) shows characteristic brain lesions, including bilateral thalamus, midbrain, internal capsule, basal ganglia, and occasionally involves an anterior horn cell. We encountered a case of a 44-year-old man who initially presented with encephalitis, which was finally diagnosed as Japanese encephalomyelitis with syringomyelia. The patient showed severe motor weakness followed by delayed recovery of functional motor activities. Cervical magnetic resonance imaging showed syrinx formation at the C5 level suggesting myelitis, and abnormal electromyographic findings were noted. Clinicians should consider the possibility that the spinal cord may be involved; an example would be syringomyelia due to myelitis in a case of JE presenting with severe and prolonged motor weakness.
Go to :

Keywords: Japanese encephalitis, Myelitis, Syringomyelia
INTRODUCTION
Japanese encephalitis (JE) is one of the common causes of epidemic encephalitis in Asia. About 20%-40% of patients who develop encephalitis die, and around half of the survivors have severe neurological sequelae [
1]. Brain magnetic resonance imaging (MRI) shows characteristic bilateral thalamic lesions involving the midbrain, internal capsule, and basal ganglia, which are associated with various clinical manifestations of movement disorders, such as Parkinsonian syndromes. Anterior horn cell (AHC) damage manifesting as focal muscle wasting and lower motor neuron type of weakness has been documented in JE [
23]. Verma et al. [
4] reported a case of acute myelitis in JE, but syringomyelia as a result of myelitis has not been reported. We experienced a quadriplegic case with a prolonged clinical course of motor recovery as a result of not only encephalitis but also syrinx formation that was assumed to occur as a result of myelitis.
Go to :

CASE REPORT
A 44-year-old male without any specific medical history visited the emergency department of our hospital with complaints of a headache and irritability that had been preceded by a fever for the past five days. After several hours, he had a generalized tonic seizure and respiratory insufficiency requiring endotracheal intubation. The cerebrospinal fluid analysis showed a white blood cell count of 45/mm3 (lymphocyte 92%), protein level of 90.1 mg/dL, and glucose level of 46 mg/dL. On neurological examination, he showed a stuporous mental status and flaccid grade 3 paralysis in both upper limbs and grade 1 in both lower limbs. We used the Medical Research Council (MRC) grading. He was generally areflexic, and there was no pyramidal tract sign.
An initial brain MRI showed patchy and subtle T2 hyperintensity of both the hippocampal gyri and left thalamus. Follow-up imaging performed on the 28th day revealed more marked and extended lesions in the bilateral hippocampal gyri, thalamus, and basal ganglia (
Fig. 1). At that time, nerve conduction studies (NCSs) showed slightly decreased amplitudes of bilateral median and ulnar compound motor action potentials (CMAPs) with normal distal latencies and conduction velocities. Furthermore, the somatosensory evoked potentials of median and tibial nerves revealed normal latencies. The diagnosis of JE was confirmed by positive JE virus IgM in enzyme-linked immunosorbent assay (ELISA) and rising titer in reverse transcription polymerase chain reaction on the 31st day and 40th day, respectively.
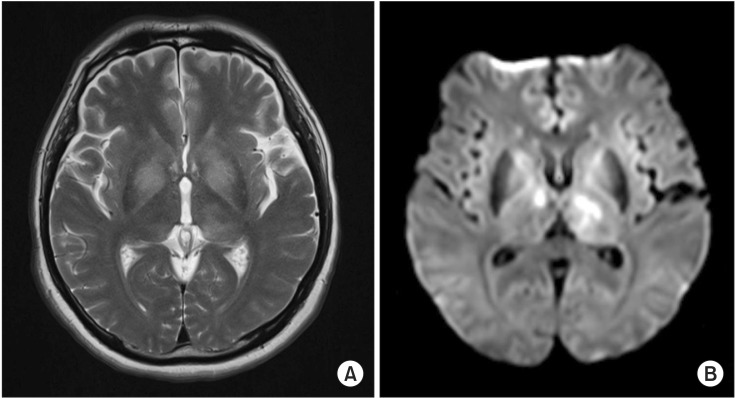 | Fig. 1Brain magnetic resonance imaging on the 28th day. (A) The axial T2-weighted image and (B) diffusion weighted image show characteristically high signal intensity in the bilateral thalamus and basal ganglia.
|
On the 62nd day, the patient was transferred to the rehabilitation unit. The motor power was grade 0 to 1 in the right upper and lower limbs, grade 2 in the left upper limb, and grade 3 in the left lower limb in terms of the MRC grading and profound muscle wasting was noted. The patient showed a mixed pattern of the deep tendon reflex (DTR) as follows: hyperactive knee jerk as opposed to hypoactive biceps, triceps and ankle jerk. His score of Modified Barthel Index (MBI) was zero.
On the 71st day, follow-up NCSs and electromyography (EMG) was performed using Medelec Synergy EMG equipment (Oxford Instrument Medical Ltd., Surrey, UK). It revealed decreased amplitudes of bilateral median CMAPs (
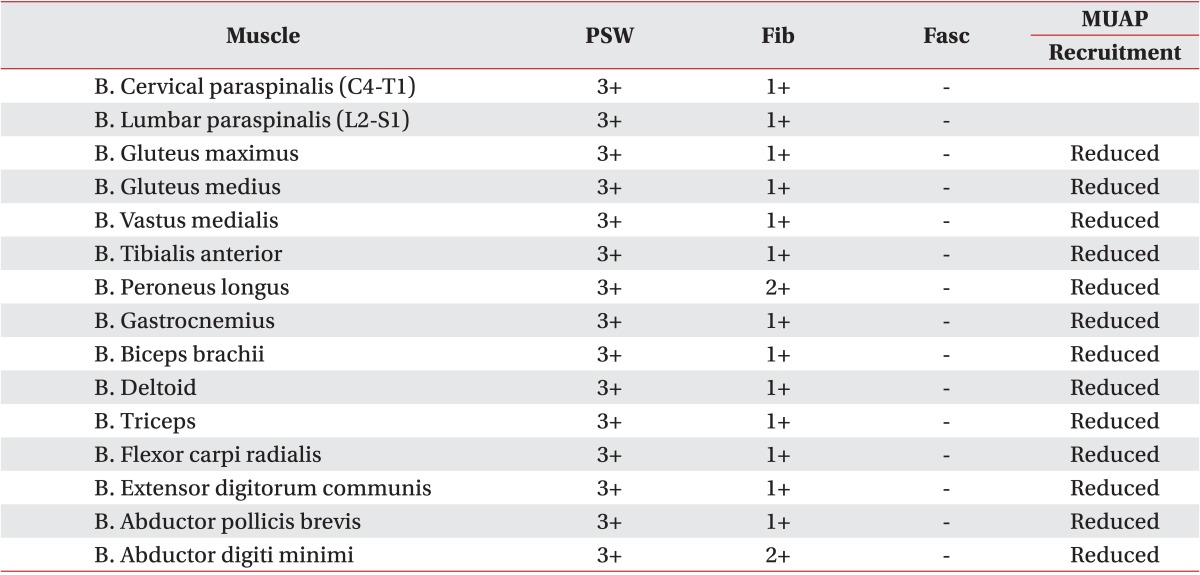
Table 1) and profuse abnormal spontaneous activities (ASAs) in all sampled muscles of the upper and lower limbs, as well as the trunk (
Table 2). The motor evoked potentials from both the abductor pollicis brevis and abductor hallucis muscles revealed decreased amplitudes of evoked potentials. A whole spine MRI on the 77th day showed syrinx formation at the C5 level of the spinal cord on the T2-weighted image (
Fig. 2). A follow-up brain MRI and diffusion tensor tractography on the 86th day demonstrated an improvement in lesions of the hippocampal gyri, thalamus, and basal ganglia. In addition, the corticospinal tract revealed preserved integrity of the tract (
Fig. 3). After physical and occupational therapy with sessions twice a day, five days a week, motor power was improved, progressing to grade 3 in the upper limbs and grade 3 to 4 in the lower limbs in terms of the MRC grading, and MBI also improved to 23 at discharge on the 104th day.
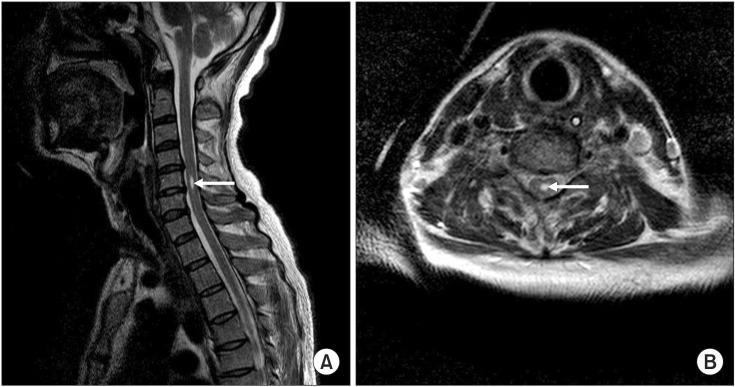 | Fig. 2Cervical magnetic resonance imaging on the 77th day. (A) Sagittal and (B) axial T2-weighted images show a cystic lesion with high signal intensity, suggesting syrinx formation (arrows) at the C5 level.
|
 | Fig. 3Brain magnetic resonance imaging and diffusion tensor tractography (DTT) on the 87th day. (A) The DTT demonstrates the preserved integrity of both corticospinal tracts. (B) The diffusion weighted image shows the regression of high signal intensity in the bilateral thalamus and basal ganglia.
|
Table 1
Findings of nerve conduction studies
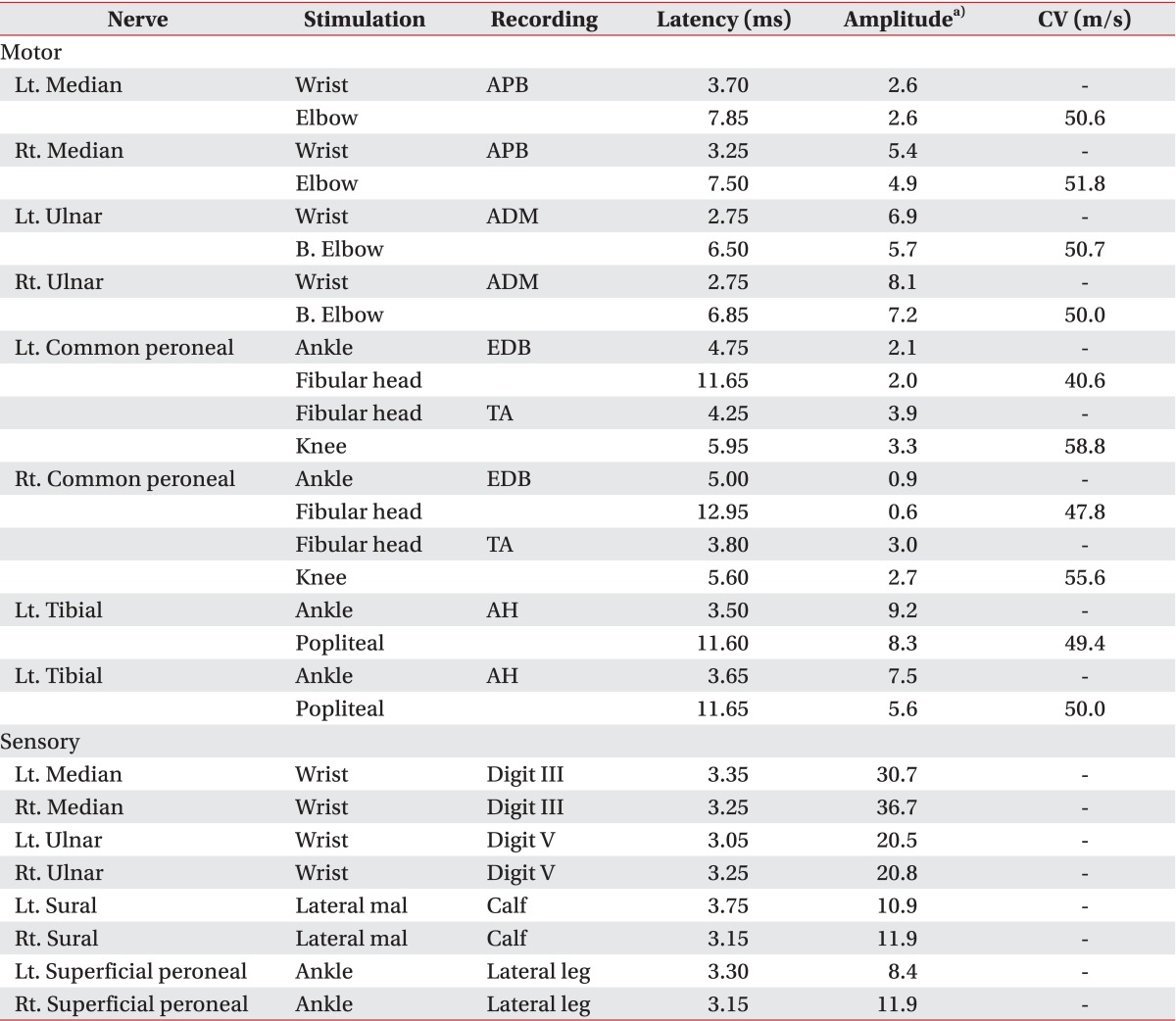

Table 2
Findings of the needle electromyography

Go to :

DISCUSSION
JE often involves the thalamus, brainstem, basal ganglia, hippocampus, and occasionally the AHCs of the spinal cord [
3]. With involvement of the AHCs, focal muscle wasting and lower motor neuron type of weakness can be seen [
3]. In this case, the patient presented with initial manifestation of flaccid paralysis and acute respiratory failure mimicking Guillain-Barre syndrome. Solomon et al. [
2] reported flaccid paralysis in JE as an initial feature in Vietnamese children. In addition, there are some reports that have demonstrated prominent lower motor neuron type of weakness resembling that in Guillain-Barre syndrome or bulbar poliomyelitis due to JE [
35].
When transferred to the rehabilitative unit, he was still in a bed-ridden state with little improvement in motor weakness. Therefore, we suspected that he had other lesions in addition to brain lesions; this suspicion was based on the clues such as profound muscle atrophy and mixed pattern of the DTR that suggested both upper and lower motor neuron lesions. There were profuse ASAs in all four limbs and trunk muscles on the EMG. Those electrodiagnostic findings were comparable to a previous report by Misra and Kalita [
3]. Therefore, MRI of the spine was performed, and it revealed syringomyelia at the C5 level, which might result from accompanying myelitis. Misra and Kalita [
3] and Kumar et al. [
6] reported increased signal changes on T2-weighted images of the spinal cord in JE patients. However, they did not report the specific location and the nature of the cord lesions.
Syringomyelia can result from a hindbrain anomaly (Chiari malformation), trauma, tumor, and arachnoiditis [
7], but we can exclude these conditions by evaluating a patient's previous medical history and imaging studies. It has been reported that parainfectious myelitis can occur after several viral infections including JE possibly due to an immune-mediated mechanism [
8], and inflammatory cord lesions, such as myelitis, may contribute to syringomyelia formation [
9]. Verma et al. [
4] reported a case with acute transverse myelitis following JE which showed diffuse high signal intensity along a cervicothoracic spinal cord on T2-weighted image. Moreover, some authors have suggested that patients with spinal cord involvement were more likely to suffer from severe diseases with poor prognosis [
23]. In addition, Solomon et al. [
1] reported persistent paralysis one to two years later in JE patients. Our patient did not show significant motor recovery, and profound motor weakness persisted for two months after the onset. The formation of syringomyelia, which may affect prolonged motor weakness, was possibly subacute rather than acute due to time-consuming pathogenesis of sustained vasogenic edema and the direct inflammatory effect on the spinal cord, although the definite timing is not clear [
9].
This case revealed that prolonged motor weakness might be attributed to syringomyelia complicated by preceding myelitis in JE. A spinal cord lesion in this case must be involved although a clinician might misunderstand JE as 'encephalitis' only-meaning brain damage. Therefore, a clinician should consider the possibility that the spinal cord may be involved in order to identify appropriate rehabilitative interventions when a patient with JE manifests unusual neurologic signs and a clinical course.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download