Abstract
Objective
To confirm functional improvement in brain tumor patients after 4-week conventional rehabilitation therapy, to compare the cognitive impairment of brain tumor patients with subacute stroke patients using computerized neuropsychological testing, and to determine the effects on functional outcomes of daily activity.
Methods
From April 2008 to December 2012, 55 patients (29 brain tumor patients and 26 subacute stroke patients) were enrolled. All patients were assessed with a computerized neuropsychological test at baseline. Motricity Index, Korean version of Mini Mental Status Examination, and Korean version of Modified Barthel Index scores were assessed at the beginning and end of 4-week rehabilitation. Conventional rehabilitation therapy was applied to both groups for 4 weeks.
Results
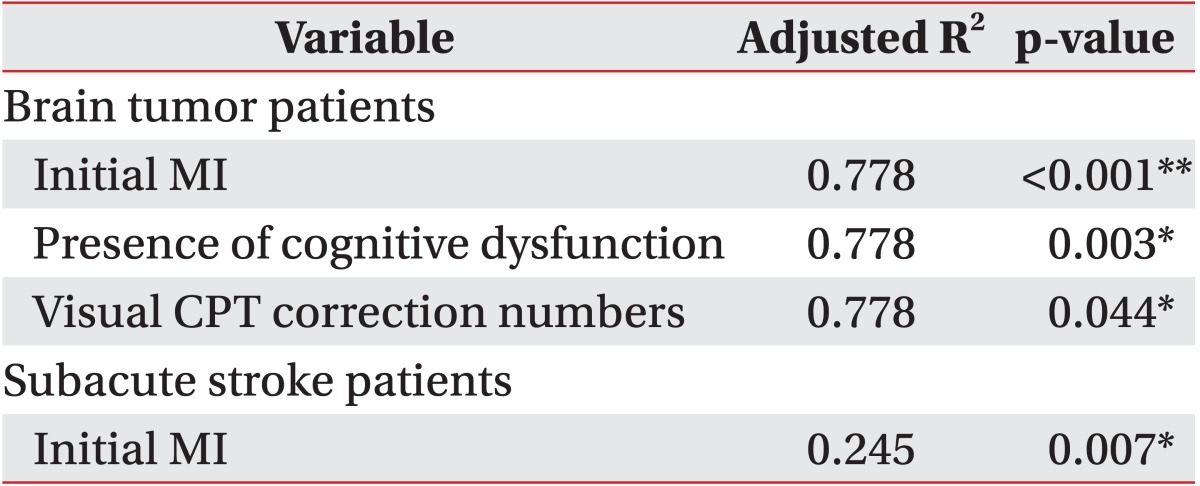
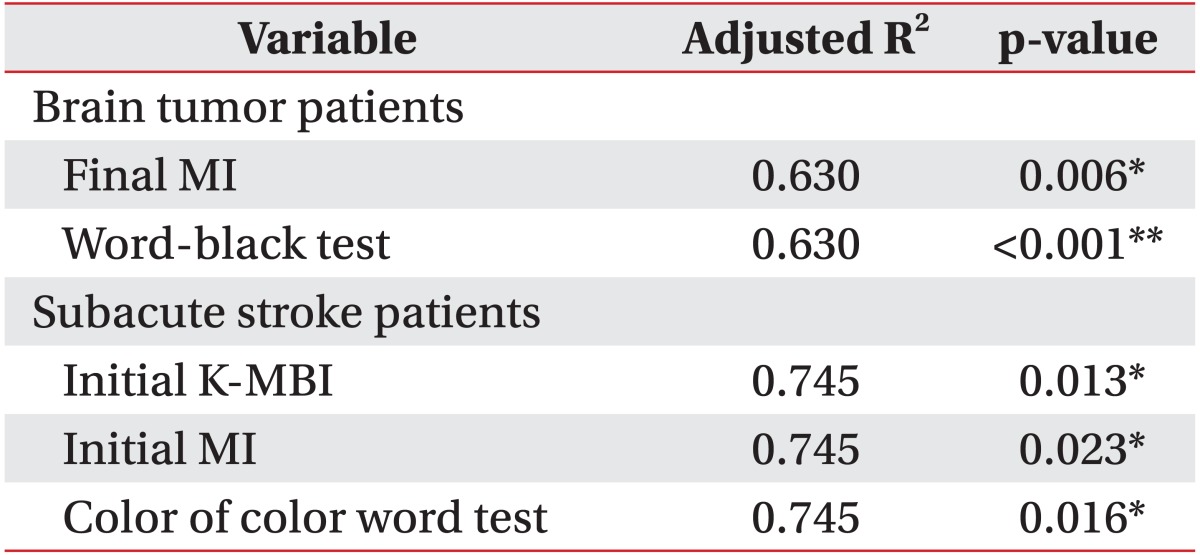
Functional outcomes of all patients in both groups significantly improved after 4-week rehabilitation therapy. In brain tumor patients, the initial Motricity Index, cognitive dysfunction, and visual continuous performance test correction numbers were strong predictors of initial daily activity function (R2=0.778, p<0.01). The final Motricity Index and word-black test were strong predictors of final daily activity function (R2=0.630, p<0.01). In patients with subacute stroke, the initial Motricity index was an independent predictor of initial daily activity function (R2=0.245, p=0.007). The initial daily activity function and color of color word test were strong predictors of final daily activity function (R2=0.745, p<0.01).
Conclusion
Conventional rehabilitation therapy induced functional improvement in brain tumor patients. Objective evaluation of cognitive function and comprehensive rehabilitation including focused cognitive training should be performed in brain tumor patients for improving their daily activity function.
Cancer is one of the most common causes of death in the world. Recent innovations in cancer treatment have led to increased life expectancies, resulting in much attention paid to cancer rehabilitation therapy over the last 3 decades [123]. However, despite improvements in therapies, brain and other central nervous system tumors are associated with poor prognosis with a 5-year survival rate of 37.5% [4], and the diagnosis of a brain tumor can produce psychological distress. Additionally, the side effects of cancer therapies and brain tissue damage from tumor growth can result in significant neurological impairments and extensive functional disabilities.
Moreover, there are several barriers to overcome in those with brain tumors. First of all, the incidence of brain tumors in Korea is relatively low at approximately 3.4 cases per 100,000 per year [5]. As a result, neurosurgeons, neurologists and neuro-oncologists may not be sufficiently aware of the benefits of rehabilitation, and they may not provide referrals for rehabilitation services [6]. Another barrier is that physiatrists may not prescribe rehabilitation therapy to brain tumor patients with very short life expectancies. Finally, there is a lack of standardized guidelines regarding appropriate rehabilitation therapy for brain tumor patients.
However, as rates of disease-free survival have increased, and interest in the long-term sequelae and the quality of life in brain tumor patients has grown, recent attention has been focused on the importance of rehabilitation of brain tumor patients. Since it is impossible to assign a control group due to ethical issues, several studies have compared brain tumor patients with various neurological conditions that do make significant functional gains following rehabilitation [7,8910111213]. In particular, as disabilities after brain tumors are similar with those after stroke for which rehabilitation has been well established, some reports have compared the functional recovery of brain tumor patients with that of stroke patients [81415]. Kim et al. [15] reported that motor weakness and impaired cognition are the most common symptoms in both groups of patients. Hence, objective evaluation of cognitive dysfunction at baseline may be important to set the rehabilitation goal. The effectiveness of cognitive assessment and rehabilitation has been shown in other types of acquired brain injuries, such as stroke and traumatic brain injury [16171819]. However, in brain tumor patients, there are few randomized controlled trials or systematic reviews about interventions addressing cognitive functioning that include a comprehensive neuropsychological evaluation or assessment of the relationship between cognitive deficits and functional rehabilitation outcomes.
The purpose of our study was to identify the functional improvement in brain tumor patients after 4-week conventional rehabilitation therapy and to compare the cognitive impairment of brain tumor patients with that of subacute stroke patients using computerized neuropsychological testing (CNT) and determine the effects on functional outcomes of daily activity.
From April 2008 to December 2012, brain tumor patients and subacute stroke patients were enrolled in this study. A brain tumor was defined as a primary or metastatic lesion detected by computed tomography (CT) or magnetic resonance imaging (MRI) and confirmed pathologically by biopsy. Stroke patients had suffered a primary ischemic or hemorrhagic stroke as revealed by CT or MRI, and were diagnosed with a first-onset stroke within 3 months of stroke onset. All patients who could follow simple commands and complete the CNT were included. Patients unable to complete a questionnaire because of aphasia, visual and/or auditory problems, neglect, apraxia, or medical instability were excluded. All patients received conventional rehabilitation therapy for 4 weeks. The study protocol was approved by our local ethics committee.
All patients were assessed using a computerized neuropsychological test (CNT; MaxMedica Inc., Seoul, Korea) immediately after they were admitted or transferred to our inpatient clinic. The test was administered by another experienced physiatrist who was blind to the study protocol. The computerized neuropsychological test consists of a forward and backward digit span test, forward and backward visual span test, verbal learning test, visual learning test, visual continuous performance test (CPT), auditory CPT, word-color test, trail making test (parts A and B), and card sorting test [20].
The digit span test evaluated auditory attention and verbal short-term memory. It recorded the longest number that a person could repeat consecutively and in reverse using numbers that were randomly generated using a computer. Visual attention and non-verbal short-term memory, which correlated with visual information, were assessed via the forward and backward visual span test. This test recorded the highest numbers of circles for which a patient could remember the sequence after each of nine circles flashed randomly onto a computer monitor.
The visual and auditory CPT tests were used to evaluate the efficiency of auditory or visual attention. Patients were asked to push a button as soon as possible after the digit '3' was presented aurally or visually during a 9-minute period, and the correction numbers and commission error were recorded by the computer. The verbal learning and visual learning tests were used to evaluate encoding and recall abilities of verbal or visual memory. The computer aurally presented a 15-item word list and visually presented another 15-item figure list over five learning trials, after which the participants were asked to recall the words or figures. The total number of recalled words or figures was calculated by the computer program.
To evaluate selective attention related to frontal executive function, especially inhibition, a word-color (Stroop) test was used, and the time taken to read the word or color of the color word was calculated. The trail making test for visuomotor coordination consisted of two parts (A and B) that were to be performed as quickly and accurately as possible. In the type A trail making test, the patient drew lines sequentially connecting, in ascending order, 25 encircled numbers randomly located on the computer monitor. In the type B trail making test, the participant had to alternate between 13 numbers and 12 Korean letters while connecting them. The score on each part represents the length of time required to complete the task [21].
The card sorting test used a number of stimulus cards. The figures on the cards were different in design, color, and quantity. Four stimulus cards were shown in the upper row of the screen, and four response cards were shown in order in the lower row. By trial and error, the participant was asked to match up each of the stimulus cards. The computer responded to the answer with 'correct' or 'wrong'. Once the participant learned to sort by one rule, after six consecutive correct responses, the initial sorting principle was changed without warning, shifting to a new principle [22].
The cognitive functions of all patients were screened using the Korean version of Mini Mental Status Examination (K-MMSE) at baseline, and patients were followed-up after a 4-week rehabilitation intervention. A score less than 24 was defined as cognitive dysfunction.
The Motricity Index (MI) was used to assess motor impairment, and the hemiparetic side score (arm score for side + leg score for side) / 2 was measured. The MI of bilateral weakness due to bi-hemispheric tumor involvement was obtained from the average of the MI of both hemiparetic sides [23]. The Korean version of the Modified Barthel Index (K-MBI) was used to measure the functional status of patients in activities of daily living (ADL) [24]. Motor and ADL functions were assessed at the beginning and end of the intervention.
Physical therapy by NDT-certified therapists was provided for 1 hour per a day, and neuromuscular electrical stimulation therapy and aerobic exercise were also applied. In addition, patients also received occupational therapy for stretching and strengthening exercises of the upper extremity and task-oriented therapy for ADL, fine motor training, and sensory motor recovery. Generally, a routine rehabilitation program was provided during 4 weeks, but computerized or focused cognitive training on neuropsychological deficits were not prescribed to either group.
All statistical analyses were performed using the SPSS for Windows statistical package ver. 12.0 (SPSS Inc., Chicago, IL, USA). A Welch t-test was used to rule out differences between the malignant and benign groups as well as between the brain tumor and stroke groups. For relevant clinical outcomes, descriptive statistics (mean±standard deviation) were computed for each assessment. Pearson correlation analysis was used to assess relationships between daily activity functions and associated factors. Multivariate linear regression analysis (backward selection) was employed to determine whether there was a significant predictable factor of daily activity function before and 4 weeks after rehabilitation. A paired t-test was used to assess the motor (MI), ADL (K-MBI), and cognitive function (K-MMSE) scores at the beginning and end of admission. For a comparison of gender, lateralization, and cognitive dysfunction, a chi-square test was used. A p-value of <0.05 was considered statistically significant.
From April 2008 to December 2012, 29 brain tumor patients (17 females) and 26 subacute stroke patients (11 females) who were admitted or transferred to the Department of Rehabilitation Medicine of Asan Medical Center in Korea were enrolled in this study.
The brain tumor group consisted of 12 men and 17 women, with a mean age of 47.9±16.8 years and a mean interval between surgery or stereotactic biopsy and time of assessment of 25.5±13.4 days. Sixteen (55.2%), 8 (27.6%), and 5 (17.2%) patients had right-sided, left-sided, and bilateral brain lesions, respectively. Thirteen patients (41.4%) had been diagnosed with benign tumors and 16 (58.6%) with malignant tumors, with brain tumor recurrence observed in 10 patients (34.5%). There were no significant intra-group differences of all parameters according to gender and lesion side in both groups.
The stroke group consisted of 15 men and 11 women, with a mean age of 64.1±12.4 years and a mean interval between stroke onset and time of assessment of 28.1±13.1 days. Eleven (42.3%), 12 (46.2%), and 3 (11.5%) patients had right-sided, left-sided, and bilateral brain lesions, respectively. Twenty patients (76.9%) had been diagnosed with ischemic stroke and 6 (23.1%) with hemorrhagic stroke.
There were no intra-group differences except for age (p<0.01) and initial K-MBI (p=0.02) (Table 1). Although there were no significant differences between the motor functions of the groups, the initial K-MBI score of the brain tumor patients was lower than that of the stroke patients (p=0.02) (Table 1).
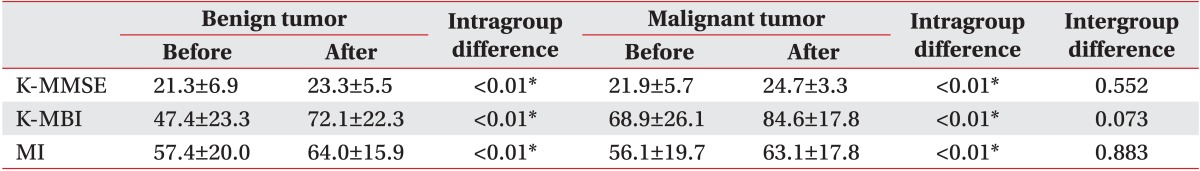
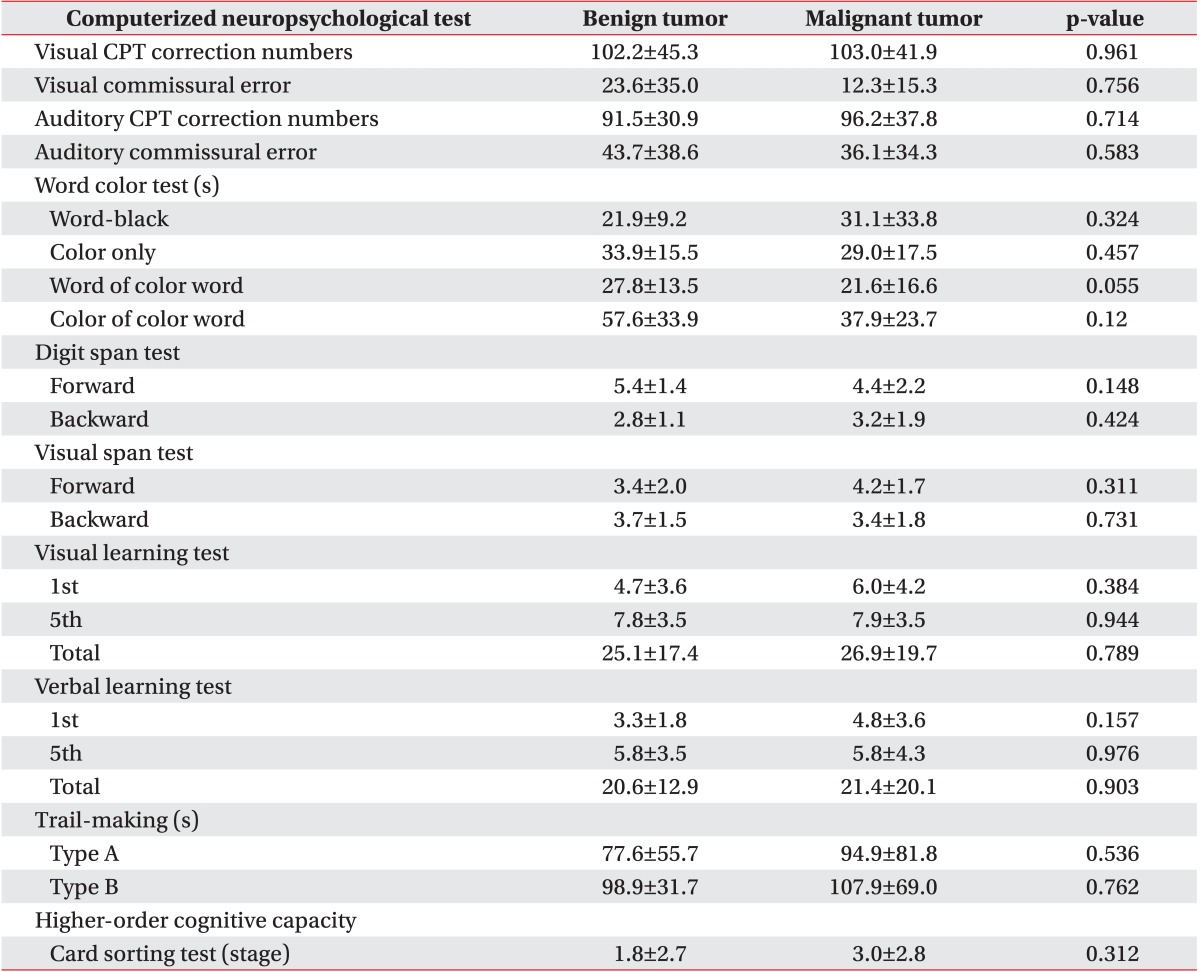
The histological findings of the malignant tumor (mean age, 43.3±15.9 years; 5 males and 8 females) and benign tumor (mean age, 47.1±18.3 years; 7 males and 9 females) groups were also compared. In the benign tumor group, 6 (46.2%), 4 (30.7%), and 3 (23.1%) patients were confirmed to have meningioma, low-grade glioma, and other tumor types, respectively. Six (46.2%), 5 (27.6%), and 5 (27.6%) patients had right-sided, left-sided, and bilateral brain lesions, respectively. In the malignant tumor group, 6 (37.6%), 5 (31.2%), and 5 (31.2%) patients were confirmed to have metastatic lesions, glioblastoma multiforme, and other tumor types, respectively. Ten (62.4%), 3 (18.8%), and 3 (18.8%) patients had right-sided, left-sided, and bilateral brain lesions, respectively. There were no intergroup differences except for the initial MI at baseline (p=0.017). All baseline variables are shown in Table 2.
All brain tumor and stroke patients significantly improved after 4 weeks of rehabilitation treatment. Scoring of parameters associated with rehabilitation outcomes is shown in Table 2.
In addition, all malignant and benign tumor patients significantly improved after 4 weeks of rehabilitation treatment. Scoring of parameters associated with rehabilitation outcomes is shown in Table 3.
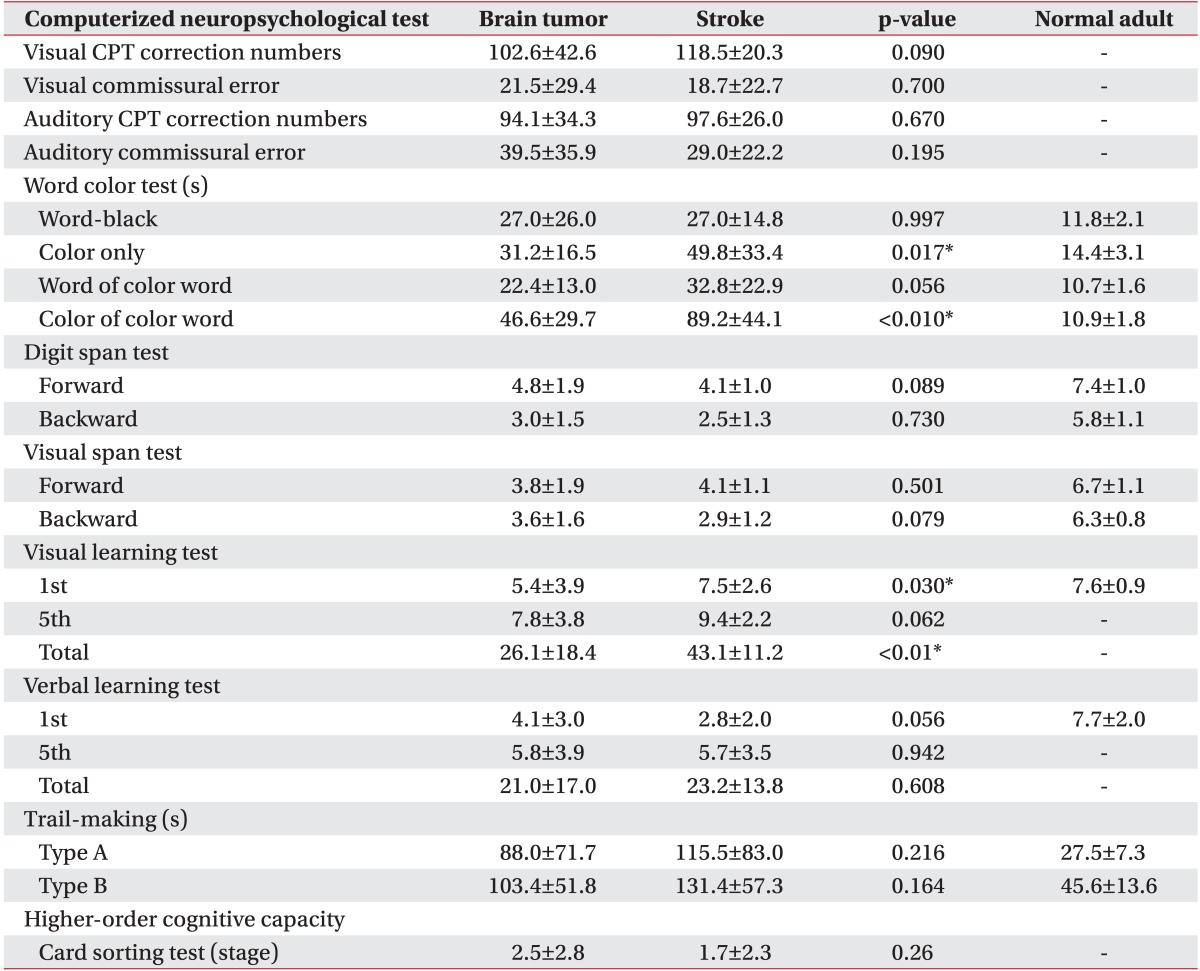
All variables of the computerized neuropsychological test in both brain tumor and stroke groups are shown in Table 4 and indicate cognitive impairment compared with normal adults. There were no statistically significant differences between the stroke and brain tumor groups in all tests except for the color only test, the color of the color word test, and the first and total visual learning tests. All variables of the computerized neuropsychological test in both benign and malignant tumor groups are shown in Table 4 and indicate cognitive impairment compared with normal adults. There were no statistically significant differences between the two groups according to tumor grade in all parameters of CNT (Table 5).
The initial K-MMSE scores of all patients correlated significantly with the subcategorized computerized neuropsychological test, except for the word of color word test, color of color word test, and trail making test type B. In patients with brain tumors, initial K-MBI scores correlated significantly with initial MI, cognitive dysfunction, visual CPT (correction numbers), word-black test, forward visual span test, and trail making test type A. Final K-MBI scores correlated significantly with initial K-MBI, final MI, word-black test, backward digit span test, and trail making test type A.
In patients with subacute stroke, initial K-MBI scores correlated significantly with initial MI. Final K-MBI scores correlated significantly with the initial K-MBI, initial and final MI, first visual learning test, color of color word test, and trail making test type A.
In our present study, cognitive dysfunction, which was assessed using objective subcategorized computerized neuropsychological testing, and its effects on initial and final rehabilitation functional outcomes were compared between brain tumor and stroke patients. These results demonstrated that impaired attention (visual) and motor and cognitive dysfunction (K-MMSE<24) affected the ADL function of brain tumor patients, and that impaired motor function affected the ADL function of stroke patients at baseline. Given that initial motor function and the number of patients with cognitive dysfunction were not statistically different between the two groups, a tendency to show poor results in the visual attention test might significantly affect the low daily activity scores of brain tumor patients at baseline. In addition, we found that after a 4-week rehabilitation, impaired selective attention (word-black test) and ADL functions at baseline and the final motor function affected final ADL function of brain tumor patients, whereas impaired selective attention (color of color word test), initial motor, and initial ADL function affected final ADL function of stroke patients.
Brain tumor patients usually suffer from cognitive impairments caused by both the tumor itself and its treatment, such as resection, chemotherapy, or radiation therapy [2526272829]. Furthermore, sensorimotor weakness and cognitive problems are common impairments in stroke and brain tumor patients [15]. However, most trials reported that the commonly used MMSE showed a lack of sensitivity to cognitive dysfunction at higher scores [303132]. In other words, an MMSE score within the supposedly normal range does not always indicate that the individual is free of significant cognitive problems, and the MMSE score alone cannot determine which cognitive domains have problems [30].
Only a few trials have assessed the cognitive function of patients with brain tumors by using comprehensive neuropsychological tests that evaluate the various cognitive domains, such as verbal and visual memory, executive functioning, attention, working memory, visuomotor coordination, and higher cognitive function [293334]. Duval et al. [33] reported a case study in which a comprehensive program for the rehabilitation of working memory and its effects on neuropsychological test performance and subjective cognitive functioning were sustained until a 3-month follow-up. Hassler et al. [34] performed neuropsychological assessments before and after intervention and observed a significant improvement in verbal learning. Gehring et al. [35] reported that a multifaceted cognitive rehabilitation program had positive effects on continued improvement in attention and verbal memory at the 6-month assessment in adult patients with lowgrade and anaplastic gliomas.
The Seoul Computerized Neuropsychological Test is a validated and objective method for comprehensive cognitive assessment with high inter-rater reliability in patients with brain injuries [23]. To the best of our knowledge, this is the first comparative study of cognitive impairment in brain tumor and stroke patients that used a computerized neuropsychological test. Although we did not follow the improvement of cognitive impairment by CNT, the majority of our patients revealed many deficits in the broad domains of attention, visual and verbal memory, visuomotor coordination, and frontal executive function at baseline, consistent with the above findings and irrespective of pathological histology. Moreover, we confirmed that attention deficits disturbed daily activity function in brain tumor patients at baseline and followup after 4 weeks and in subacute stroke patients at follow-up after 4 weeks. Therefore, attention should be paid to the cognitive as well as functional status of patients with brain tumors and stroke. In addition, not only conventional rehabilitation therapy, but also cognitive training focusing on specific deficits should be prescribed.
This study had several limitations, of which the most serious is the small number of patients evaluated. Thus, monitoring the effects of each treatment, including tumor resection, chemotherapy and radiotherapy, that might cause cognitive impairment could not be performed [36]. However, because many of the brain tumor patients had received resection and radiotherapy or chemotherapy simultaneously, there limitations might be attributed to clinical situations. Another limitation is the heterogeneity of lesion sites and tumor histology. Although malignant tumors were compared with benign tumors, further studies with a larger number of patients and groups subcategorized by histological type should be conducted. Third, several factors, including age and K-MBI, that showed statistical differences could also have an effect on the functional outcome after rehabilitation therapy. Although these factors were not correlated with final daily activity in regression analysis, this result should be proven in a large-scaled prospective study. Fourth, only cognitive status was assessed by the K-MMSE at discharge in the absence of follow-up computerized neuropsychological testing at discharge. As mentioned previously, a stable MMSE score over time does not necessarily indicate a stable cognitive function lacking significant changes [30]. Fifth, the brain tumor patients of this study could not be compared with brain tumor patients who did not perform rehabilitation therapy since it was impossible to assign patients to control group due to ethical reasons. Finally, a therapeutic intervention period of 4 weeks might be too short to demonstrate full recovery of patients with severe disability after stroke or brain tumor treatment since functional recovery of a stroke or brain tumor could be considered to require 6 months after onset [3738].
Further investigations, with larger numbers of patients, follow-up computerized neuropsychological testing, and long-term follow-up are warranted to assess the effects of cognitive training on cognitive and functional outcomes in brain tumor patients in terms of the brain lesion and tumor histology type.
In conclusion, patients with subacute stroke and patients with brain tumors reveal deficits in the broad domains of attention, visual and verbal memory, visuomotor coordination, and frontal executive function. In particular, attention deficits of patients with brain tumors affect functional outcomes both at baseline and after a 4-week intervention. Therefore, an objective cognitive evaluation should be performed in brain tumor patients for improving their daily activity function and quality of life.
References
1. DeLisa JA. A history of cancer rehabilitation. Cancer. 2001; 92(4 Suppl):970–974. PMID: 11519022.

2. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development, and evaluation of a model of care. Arch Phys Med Rehabil. 1978; 59:410–419. PMID: 687056.
3. Harvey RF, Jellinek HM, Habeck RV. Cancer rehabilitation: an analysis of 36 program approaches. JAMA. 1982; 247:2127–2131. PMID: 7062528.

4. Jung KW, Yoo H, Kong HJ, Won YJ, Park S, Lee SH. Population-based survival data for brain tumors in Korea. J Neurooncol. 2012; 109:301–307. PMID: 22660961.

5. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14. PMID: 23613665.

6. Kirshblum S, O'Dell MW, Ho C, Barr K. Rehabilitation of persons with central nervous system tumors. Cancer. 2001; 92(4 Suppl):1029–1038. PMID: 11519030.

7. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000; 79:327–335. PMID: 10892618.
8. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998; 79:1386–1390. PMID: 9821898.

9. Huang ME, Wartella JE, Kreutzer JS. Functional outcomes and quality of life in patients with brain tumors: a preliminary report. Arch Phys Med Rehabil. 2001; 82:1540–1546. PMID: 11689973.

10. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998; 79:1530–1534. PMID: 9862294.
11. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006; 85:568–573. PMID: 16788387.
12. Sherer M, Meyers CA, Bergloff P. Efficacy of postacute brain injury rehabilitation for patients with primary malignant brain tumors. Cancer. 1997; 80:250–257. PMID: 9217038.

13. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001; 82:457–463. PMID: 11295004.

14. Geler-Kulcu D, Gulsen G, Buyukbaba E, Ozkan D. Functional recovery of patients with brain tumor or acute stroke after rehabilitation: a comparative study. J Clin Neurosci. 2009; 16:74–78. PMID: 19022673.

15. Kim HJ, Kim DY, Chun MH, Lee SJ. Functional outcomes and characteristics of patients with brain tumors after inpatient rehabilitation: comparison with ischemic stroke. J Korean Acad Rehabil Med. 2010; 34:290–296.
16. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10:1037–1044. PMID: 19801201.

17. Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, et al. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000; 6:299–312. PMID: 10824502.

18. Sohlberg MM, McLaughlin KA, Pavese A, Heidrich A, Posner MI. Evaluation of attention process training and brain injury education in persons with acquired brain injury. J Clin Exp Neuropsychol. 2000; 22:656–676. PMID: 11094401.
19. Winkens I, Van Heugten CM, Wade DT, Fasotti L. Training patients in Time Pressure Management, a cognitive strategy for mental slowness. Clin Rehabil. 2009; 23:79–90. PMID: 19114440.

20. Kim YH, Shin SH, Park SH, Ko MH. Cognitive assessment for patient with brain injury by computerized neuropsychological test. J Korean Acad Rehabil Med. 2001; 25:209–216.
21. Perianez JA, Rios-Lago M, Rodriguez-Sanchez JM, Adrover-Roig D, Sanchez-Cubillo I, Crespo-Facorro B, et al. Trail Making Test in traumatic brain injury, schizophrenia, and normal ageing: sample comparisons and normative data. Arch Clin Neuropsychol. 2007; 22:433–447. PMID: 17336493.

22. Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Selective neuronal damage and Wisconsin Card Sorting Test performance in atherosclerotic occlusive disease of the major cerebral artery. J Neurol Neurosurg Psychiatry. 2011; 82:150–156. PMID: 20802218.

23. Kim YH, Ko MH, Seo JH, Park SH, Kim KS, Jang EH, et al. Effect of computer-assisted cognitive rehabilitation program for attention training in brain injury. J Korean Acad Rehabil Med. 2003; 27:830–839.
24. Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, et al. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007; 31:283–297.
25. Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006; 24:1305–1309. PMID: 16525186.

26. Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003; 5:89–95. PMID: 12672280.

27. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000; 47:324–334. PMID: 10942005.

28. Gehring K, Aaronson NK, Gundy CM, Taphoorn MJ, Sitskoorn MM. Predictors of neuropsychological improvement following cognitive rehabilitation in patients with gliomas. J Int Neuropsychol Soc. 2011; 17:256–266. PMID: 21205412.

29. Gehring K, Aaronson NK, Taphoorn MJ, Sitskoorn MM. Interventions for cognitive deficits in patients with a brain tumor: an update. Expert Rev Anticancer Ther. 2010; 10:1779–1795. PMID: 21080804.

30. Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003; 21:3557–3558. PMID: 12913103.

31. Fox SW, Mitchell SA, Booth-Jones M. Cognitive impairment in patients with brain tumors: assessment and intervention in the clinic setting. Clin J Oncol Nurs. 2006; 10:169–176. PMID: 16708701.

32. Kehayov II, Kitov BD, Zhelyazkov CB, Raykov SD, Davarski AN. Neurocognitive impairments in brain tumor patients. Folia Med (Plovdiv). 2012; 54:14–21. PMID: 23441465.

33. Duval J, Coyette F, Seron X. Rehabilitation of the central executive component of working memory: a reorganisation approach applied to a single case. Neuropsychol Rehabil. 2008; 18:430–460. PMID: 18576271.

34. Hassler MR, Elandt K, Preusser M, Lehrner J, Binder P, Dieckmann K, et al. Neurocognitive training in patients with high-grade glioma: a pilot study. J Neurooncol. 2010; 97:109–115. PMID: 19763790.

35. Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009; 27:3712–3722. PMID: 19470928.

36. Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998; 25(1 Suppl 1):39–47. PMID: 9482539.
37. Khan F, Amatya B, Drummond K, Galea M. Effectiveness of integrated multidisciplinary rehabilitation in primary brain cancer survivors in an Australian community cohort: a controlled clinical trial. J Rehabil Med. 2014; 46:754–760. PMID: 24940656.

38. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011; 377:1693–1702. PMID: 21571152.

Table 2
Functional outcomes of benign tumor and malignant tumor groups before and after 4 weeks of rehabilitation therapy according to tumor grade

Table 3
Functional outcomes between brain tumor and stroke groups before and after 4 weeks of rehabilitation therapy




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download