1. Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002; 83:1629–1637. PMID:
12422337.

2. Afifi AK, Bergman RA. Functional neuroanatomy: text and atlas. 2nd ed. New York: McGraw-Hill;2005. p. 59–60.
3. Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med. 2014; 46:193–199. PMID:
24531325.

4. Davidoff RA. The pyramidal tract. Neurology. 1990; 40:332–339. PMID:
2405296.

5. Jang SH. The role of the corticospinal tract in motor recovery in patients with a stroke: a review. NeuroRehabilitation. 2009; 24:285–290. PMID:
19458437.

6. York DH. Review of descending motor pathways involved with transcranial stimulation. Neurosurgery. 1987; 20:70–73. PMID:
3543726.

7. DeVetten G, Coutts SB, Hill MD, Goyal M, Eesa M, O'Brien B, et al. Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke. 2010; 41:751–756. PMID:
20203322.

8. Bembenek JP, Kurczych K, Karli Nski M, Czlonkowska A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke: a systematic review of the literature. Funct Neurol. 2012; 27:79–84. PMID:
23158578.
9. van Kuijk AA, Pasman JW, Hendricks HT, Zwarts MJ, Geurts AC. Predicting hand motor recovery in severe stroke: the role of motor evoked potentials in relation to early clinical assessment. Neurorehabil Neural Repair. 2009; 23:45–51. PMID:
18794218.

10. Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: a review. NeuroRehabilitation. 2010; 27:367–372. PMID:
21160127.

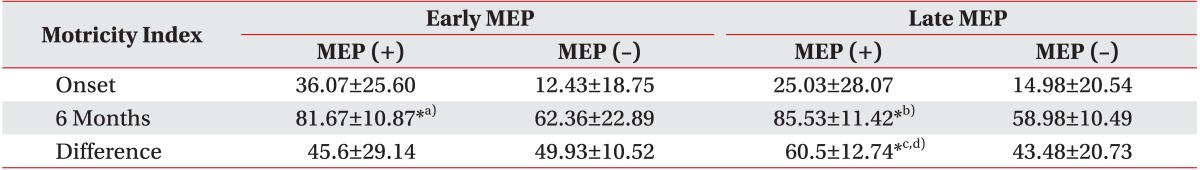
11. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985; 1:1106–1107. PMID:
2860322.

12. Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006; 117:1641–1659. PMID:
16595189.

13. Catano A, Houa M, Caroyer JM, Ducarne H, Noel P. Magnetic transcranial stimulation in acute stroke: early excitation threshold and functional prognosis. Electroencephalogr Clin Neurophysiol. 1996; 101:233–239. PMID:
8647036.

14. Pizzi A, Carrai R, Falsini C, Martini M, Verdesca S, Grippo A. Prognostic value of motor evoked potentials in motor function recovery of upper limb after stroke. J Rehabil Med. 2009; 41:654–660. PMID:
19565160.

15. Rapisarda G, Bastings E, de Noordhout AM, Pennisi G, Delwaide PJ. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke. 1996; 27:2191–2196. PMID:
8969779.

16. Timmerhuis TP, Hageman G, Oosterloo SJ, Rozeboom AR. The prognostic value of cortical magnetic stimulation in acute middle cerebral artery infarction compared to other parameters. Clin Neurol Neurosurg. 1996; 98:231–236. PMID:
8884095.

17. Vang C, Dunbabin D, Kilpatrick D. Correlation between functional and electrophysiological recovery in acute ischemic stroke. Stroke. 1999; 30:2126–2130. PMID:
10512917.

18. Butler JA. How comparable are tests of apraxia? Clin Rehabil. 2002; 16:389–398. PMID:
12061473.

19. Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980; 19:382–389. PMID:
7439211.

20. Kwon YH, Jeoung YJ, Lee J, Son SM, Kim S, Kim C, et al. Predictability of motor outcome according to the time of diffusion tensor imaging in patients with cerebral infarct. Neuroradiology. 2012; 54:691–697. PMID:
22015644.

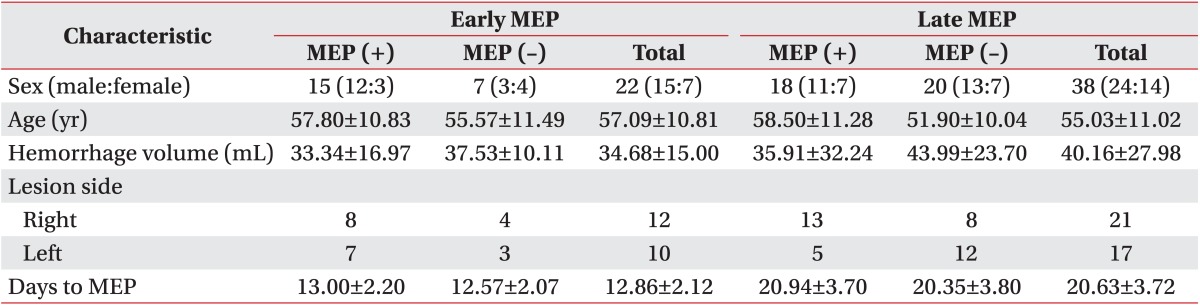
21. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–1305. PMID:
8711791.

22. Inaji M, Tomita H, Tone O, Tamaki M, Suzuki R, Ohno K. Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir Suppl. 2003; 86:445–448. PMID:
14753483.

23. Jang SH, Byun WM, Han BS, Park HJ, Bai D, Ahn YH, et al. Recovery of a partially damaged corticospinal tract in a patient with intracerebral hemorrhage: a diffusion tensor image study. Restor Neurol Neurosci. 2006; 24:25–29. PMID:
16518025.
24. Yang DS, Kim DS, Kim YH, Jang SH. Demonstration of recovery of a severely damaged corticospinal tract: a diffusion tensor tractography and transcranial magnetic stimulation follow-up study. J Comput Assist Tomogr. 2008; 32:418–420. PMID:
18520549.
25. Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996; 40:216–226. PMID:
8773603.

26. Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998; 11:655–662. PMID:
9870133.

27. Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008; 63:272–287. PMID:
18383072.

28. Arac N, Sagduyu A, Binai S, Ertekin C. Prognostic value of transcranial magnetic stimulation in acute stroke. Stroke. 1994; 25:2183–2186. PMID:
7974543.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download