INTRODUCTION
Various methods, such as oropharyngeal exercise, compensation maneuvers, diet control and electrical stimulation, are used to improve swallowing in patients with dysphagia. Recently, interest in the use of electrical stimulation for the treatment of dysphagia has increased and many researchers have been conducting studies related to this [
1]. The positions and types of electrodes used for electrical stimulation include intraoral, transcutaneous, and intramuscular [
23]. However, not all types of electrical stimulation are used for treatment. The VitalStim (Chattanooga Group, Hixson, TN, USA), a transcutaneous type of electrical stimulation that received approval from the US Food and Drug Administration, is one of the methods used [
4].
In recent years, several studies have focused attention on electrical stimulation of hyolaryngeal muscles. Freed et al. [
4] published data on the application of transcutaneous electrical stimulation to a group of 63 stroke patients and compared the data to that of 36 stroke patients treated with thermal tactile stimulation. The results of their study demonstrated a significant improvement in the electrical stimulation group. However, the study has been criticized for its many methodological flaws, including the lack of a clearly articulated physiologic rationale guiding the site of stimulation, the subject eligibility criteria and failures to control for spontaneous recovery, randomization, the validity of the measure used to determine outcomes and experimenter bias [
5]. There were several studies following the Freed et al. [
4] study that compared transcutaneous electrical stimulation to traditional swallowing therapy. Blumenfeld et al. [
6] investigated the efficacy of electrical stimulation in treating persons with dysphagia and aspiration. Their results showed that dysphagia therapy with electrical stimulation was superior to traditional dysphagia therapy alone. However, Kiger et al. [
7] reported no statistically significant difference in outcomes in patients treated with transcutaneous electrical stimulation therapy and those that received traditional swallowing therapy for dysphagia.
Various muscle complexes are involved in the swallowing process. The suprahyoid muscles include the mylohyoid, geniohyoid and the anterior belly of the digastrics, while the infrahyoid muscles include the thyrohyoid, sternohyoid and omohyoid. The suprahyoid and thyrohyoid muscles move the hyoid bone in an anterosuperior direction. However, the other infrahyoid muscles move the hyoid bone in an inferior direction [
1]. Contraction of the hyolaryngeal muscles is one of the first events in the swallowing reflex. This pulls the larynx in the anterosuperior direction and facilitates epiglottis rotation, helping prevent aspiration while the bolus passes through the pharynx [
8]. Combined stimulation of both the suprahyoid and infrahyoid muscles, located in the submental area and the anterior throat areas, is standard practice with transcutaneous electrical stimulation [
9]. Using transcutaneous electrical stimulation, a tolerable level of electrical stimulation at the submental area moves the hyoid bone in an anterosuperior direction, regardless of the muscle depth of the stimulation. However, electrical stimulation at the throat area moves the hyoid bone in an inferior direction if insufficient current is flowing to the deep muscles, including the thyrohyoid muscle [
10].
Over the past few years, a considerable number of studies have analyzed hyolaryngeal structural movements during swallowing with simultaneous electrical stimulation. Humbert et al. [
11] found that significant laryngeal and hyoid descent occurred with stimulation at rest in 28 healthy volunteers. Also, during stimulated swallowing, significant reductions in the peak elevation of both the larynx and hyoid bone occurred. Ludlow et al. [
12] examined 11 participants with chronic long standing dysphagia. They were interested in the effect of electrical stimulation on the position of the hyoid bone under conditions of no stimulation, low sensory-level stimulation, maximally tolerated motor level stimulation during swallowing and at rest. They showed that significant hyoid depression occurred only during stimulated swallowing and at rest. They also showed that participants who had reduced aspiration and penetration during swallowing with stimulation had greater hyoid depression during stimulation. Therefore, they concluded that transcutaneous electrical stimulation would be detrimental to hyolaryngeal elevation in dysphagic individuals. And they suggested that patients should be carefully screened prior to the use of transcutaneous electrical stimulation to determine whether they would be placed at increased risk of aspiration.
Although some studies showed that transcutaneous electrical stimulation can depress the hyoid bone in both healthy people and patients with dysphagia, transcutaneous electrical stimulation is still used as a treatment option for patients with dysphagia without proven aspiration and penetration safety. The VitalStim (Chattanooga Group) is a transcutaneous method of electrical stimulation that received approval from the US Food and Drug Administration. However, as mentioned above, there has been some controversy in recent studies over the efficacy of electrical therapy for dysphagia. Therefore, there is a real need to evaluate the efficacy of continuous electrical therapy for dysphagia.
An additional study revealed that transcutaneous electrical stimulation of the infrahyoid muscles for 2 weeks, along with effortful swallowing, had a significant effect on the maximal vertical displacement of the larynx [
13]. However, this study investigated the kinematic result of treatment on repeated sessions of hyolaryngeal electrical stimulation without a kinematic change analysis during stimulation. Although, many clinicians believe that transcutaneous electrical stimulation along with effortful swallowing has a strengthening effect on patients with dysphagia, we still do not know whether transcutaneous electrical stimulation is detrimental for patients with dysphagia.
We addressed two questions in our study: 1) Does transcutaneous electrical stimulation with effortful swallowing really reduce the elevation of the larynx and hyoid bone? If transcutaneous electrical stimulation with effortful swallowing really does reduce elevation, what is the therapeutic mechanism underlying transcutaneous electrical stimulation in patients with dysphagia? 2) Do different arrangements of the surface electrode induce different contractions of the hyolaryngeal muscles that result in different levels of hyolaryngeal elevation?
Therefore, to assess the kinematic effect and safety during electrical stimulation with effortful swallowing, we measured the motion of the hyoid and larynx using various placements of surface electrodes in healthy volunteers. We analyzed the movement of the hyoid and larynx according to temporal change.
MATERIALS AND METHODS
Participants
To conduct this prospective study, it was necessary to plan the number of subjects needed. Thus, we determined the number of subjects using a calculation method developed by the Occupational and Environmental Medicine Department at the Kosin University College of Medicine and received the approval of the Institutional Review Board. The level of significance was set as α=0.05 and type II error (β) was set at 0.20, while the power of the test was maintained at 80% based on the results of a preliminary study [
14]. The results of a preliminary study with 40 healthy volunteers showed that the average horizontal movement of the hyoid bone was 12.55 mm (standard deviation, 2.59 mm). Considering the fact that there was a difference of 20% in the horizontal movement of the hyoid bone with a posture of withdrawn jaw compared to when the jaw was extended, the variation of the hyoid bone according to the posture of the patients was assumed to be significant. Thus, the minimum number of subjects required for outcome measurements in the movement of the hyoid bone was determined to be 18. Considering a dropout rate of 10%, 18-20 subjects were needed to conduct this study. Participants were recruited using advertisements at the Dong-A University. Twenty healthy volunteers (11 males and 9 females), with an average age of 21.4 years, participated in the study. None of the subjects was on regular medication or had any history of neurological, phonological, psychiatric, speech, or swallowing disorders. An informed consent form approved by the Institutional Review Board at the Kosin University College of Medicine in the Republic of Korea (IRB protocol number: 11-44) was completed by each subject prior to the study. All the subjects were educated about the study purpose, design, safety and potential complications.
Electrical stimulation
The skin in the submental and laryngeal regions was cleaned with alcohol to increase the adherence of the electrodes to the skin. All male participants were shaved to improve adherence of the electrodes to the skin. Adult sized electrodes (VitalStim, REF 59000) with a 2.1 cm round active area were used. Each participant was familiarized with the expected sensations upon use of the surface electrical stimulation unit (VitalStim). The electrical stimulation unit provided two channels of bipolar electrical stimulation at a pulse rate of 80 Hz and a fixed biphasic pulse duration of 700 µs. Each channel could be independently adjusted between 0 and 25 µA of stimulation intensity.
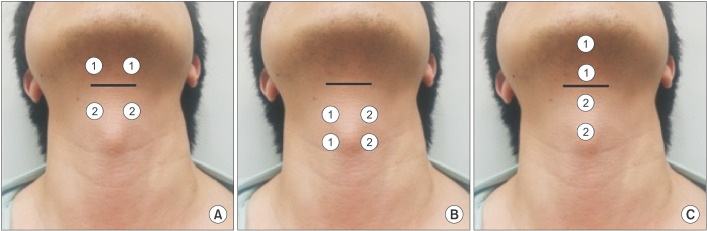
A total of 3 different electrode placements were used (
Fig. 1). In placement I, two sets of electrode pairs were placed in the suprahyoid and infrahyoid areas (SI) targeting the suprahyoid and infrahyoid muscles. In placement II, two sets of electrode pairs were placed in only the infrahyoid area (IO) targeting the thyrohyoid and sternohyoid muscles. In placement III, two sets of electrode pairs were placed vertically in the suprahyoid and infrahyoid areas (SIV). Before starting a recording, each electrode pair was placed on the skin, and the stimulation intensity was gradually increased until the participants could feel a tugging sensation. The intensity level was then increased further until the participants felt that any additional increase would not be sensed, yielding the maximum tolerance level. This maximum tolerance level was used and recorded for all electrode pairs.
Videofluoroscopic swallowing study
Five milliliters of fluid, containing barium diluted to 1:10 with normal saline, was given to each participant. Initially, the participant swallowed with no electrical stimulation and the results were recorded. During swallowing without stimulation, electrodes were placed on the proper area (SI, IO, SIV). Electrical stimulation was then applied and the maximum tolerance level was determined and recorded. After we determined the maximum tolerance level, participants were asked to start swallowing while in a seated position. Stimulation was initiated and maintained before, during, and after the swallowing. For stimulation at rest trials, participants were instructed not to move and to keep their jaws closed. This was done to prevent jaw opening because of the proximity of the surface electrodes to the anterior belly of the digastric muscle, which overlies the mylohyoid in the suprahyoid area.
VFSS was conducted separately using the 3 electrode stimulation placements (SI, IO, SIV) and included 6 total swallows with and without stimulation. To prevent muscle fatigue, there was a 10-minute rest period between each trial. All processes were recorded and each video was trimmed and digitized for motion analysis. The video recordings were performed to satisfy three conditions. First, video recordings had to include the time period when the base of the tongue started pushing the food bolus at the pharyngeal cavity to the point when the tail of the bolus passed through the cricopharyngeal muscles. Second, based on the anatomical position, the recording area included the posterior half of the hard palate anterior, the anterior edge of the lower endplate of the second and fourth cervical vertebrae at the posterior, the lower part of the cricopharyngeal muscles at the inferior, and the hard palate at the superior. Lastly, the videos were recorded for clear visualization of the movement of hyolaryngeal structures.
Kinematic analysis
For motion analysis, recorded videos were trimmed with the Pinnacle 12.0 program. We digitized the position of the anatomical structures and the food bolus using the Ariel Performance Analysis System (APAS; Ariel Dynamics Inc., Trabuco Canyon, CA, USA), which we had previously used for gait analyses, and analyzed the movement of the anatomical structures and the food bolus at 30 frames per second. The coordinate axes were determined as in
Fig. 2. The zero point was defined as the anterior-inferior margin of the fourth cervical vertebral body. The vertical axis was defined as the straight line connecting the zero point with the anterior-inferior margin of the second cervical vertebral body. Consequently, the horizontal axis was perpendicular to the vertical axis at the zero point. The anterior-inferior margin of the hyoid bone and the anterior edge of the vocal cord were marked for the motion analysis on the coordinate axes. APAS displayed the essential structures of deglutition, including the maxillary bone, mandible bone, cervical vertebrae, arytenoids cartilage, epiglottis, hyoid bone, and vocal cord. The values calculated from these processes were represented as graphs using MATLAB (MathWorks, Natick, MA, USA).
Parameter measures
The parameters obtained by kinematic analysis are horizontal initial point (HIP), the horizontal position of the structure before swallowing compared to the zero point; vertical initial point (VIP), the vertical position of the structure before swallowing compared to the zero point; horizontal peak point (HPP), the horizontal peak point of the structure during swallowing; vertical peak point (VPP), the vertical peak point of the structure during swallowing; horizontal distance (HD) and vertical distance (VD) of the hyoid bone and the vocal cord from the coordinate axes. The HD was calculated from the initial point (IP) to the maximally displaced points of the structures in the vertical axis during swallowing. The VD was calculated from the initial points to the maximally displaced points of the structures in the horizontal axis during swallowing.
Statistical analysis
A paired t-test was used to evaluate the significances of the measured parameters with and without stimulation. Values were considered to be statistically significant if the p-value was less than 0.05. Statistical analyses of all data was conducted using SPSS ver. 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
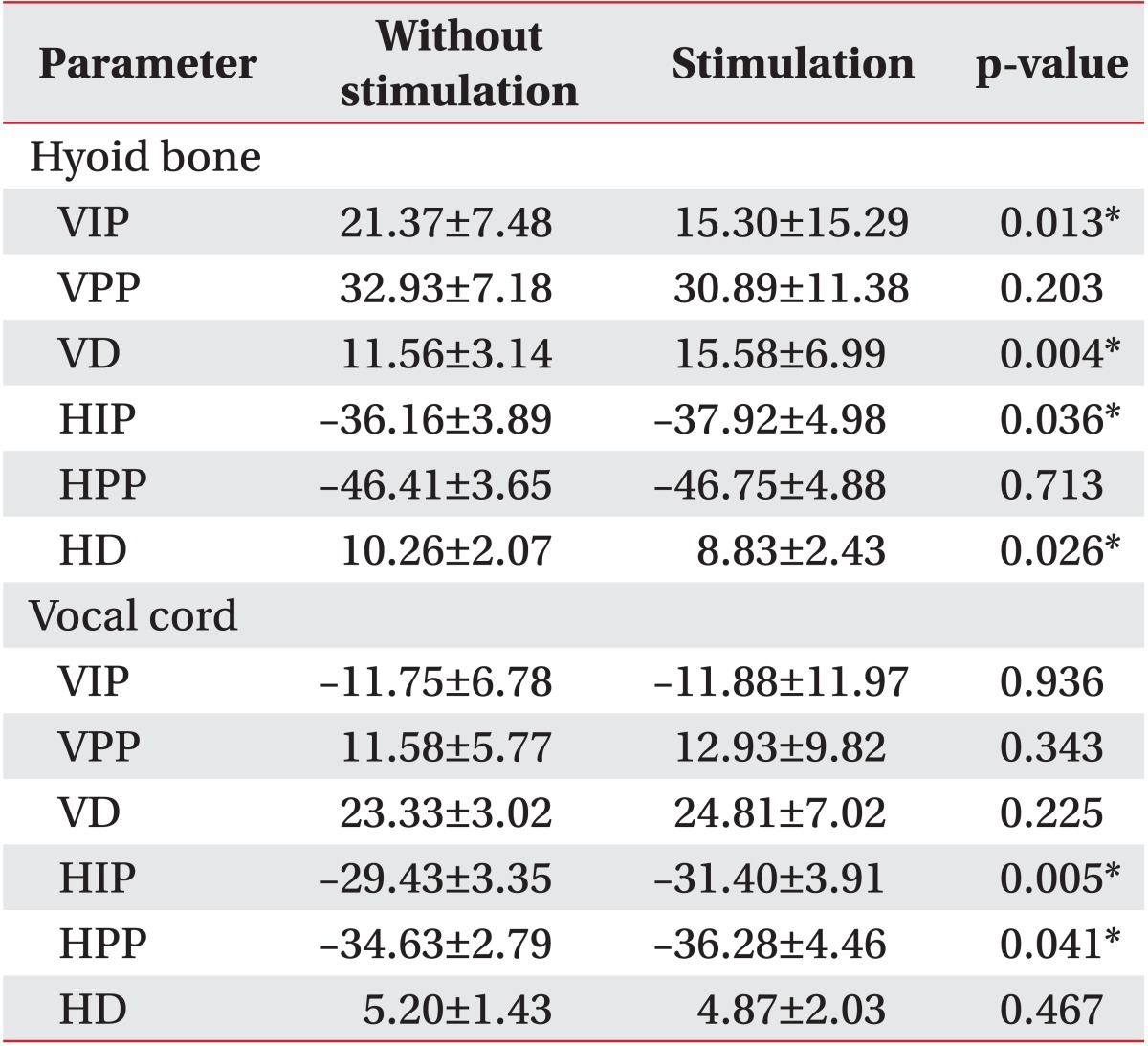
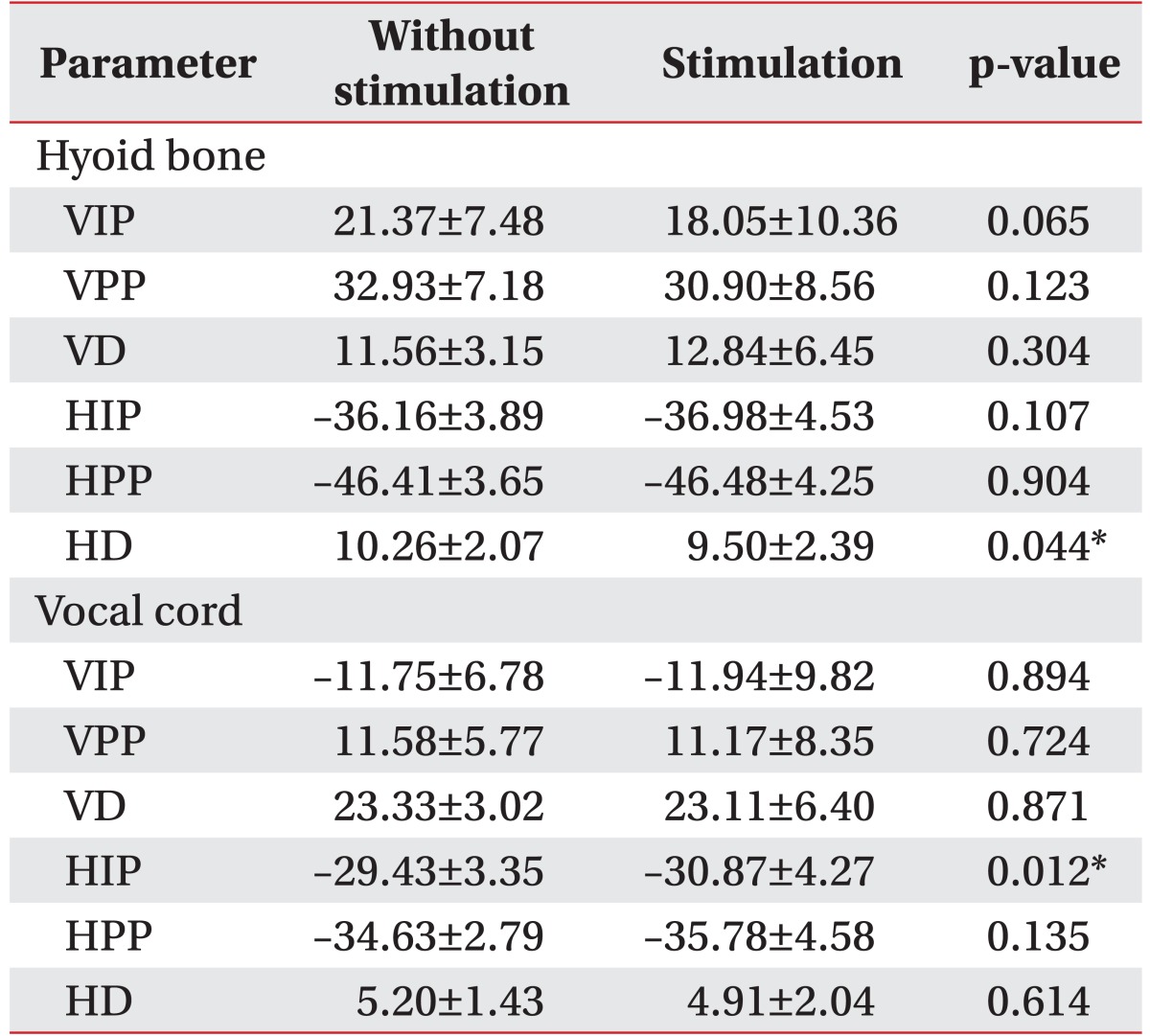
Vertical and horizontal movements of the hyoid bone and vocal cord with and without electrical stimulation on the suprahyoid and infrahyoid muscles (SI).
Initially, different results were observed for the movement of the hyoid bone between trials with and without stimulation during swallowing (
Table 1).
The VIP of the hyoid bone showed significantly more downward displacement with stimulation compared to without stimulation (p<0.05) and the VD was significantly larger (p<0.05). The VPP reached almost the same position in both trials. The HIP of the hyoid bone showed significantly more anterior displacement with stimulation compared to without stimulation (p<0.05) whereas the HD was smaller (p<0.05). The HPP was almost the same in both trials.
Vertical vocal cord movement showed no definitive differences between trials with and without stimulation during swallowing (
Table 1). However, analysis of horizontal vocal cord movement resulted in some significant differences in findings. The HIP of the vocal cord showed significantly more anterior displacement with stimulation compared to without stimulation, and the HPP of the vocal cord also showed significantly greater anterior displacement with stimulation (p<0.05). However, the HD with stimulation was smaller than without stimulation (p<0.05).
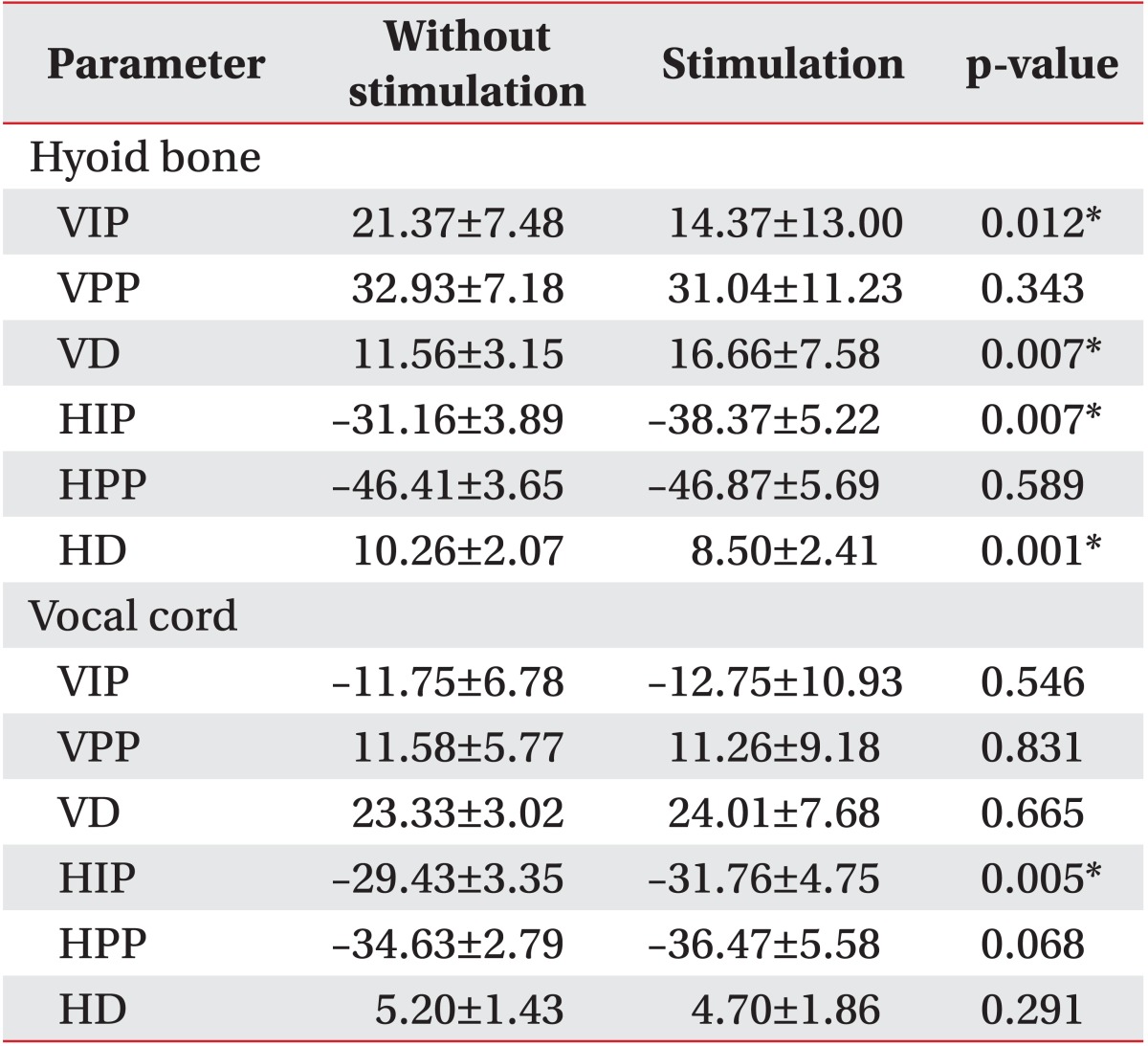
Vertical and horizontal movements of the hyoid bone and vocal cord with and without stimulation on only the infrahyoid muscles (IO).
Hyoid movement showed different results with and without stimulation during swallowing (
Table 2).
The VIP of the hyoid bone with stimulation showed significantly more downward displacement compared to without stimulation (p<0.05) and the VD with stimulation was significantly larger (p<0.05). The VPP reached almost the same position in both trials. The HIP of the hyoid bone showed significantly more anterior displacement with stimulation (p<0.05), but the HD with stimulation was smaller (p<0.05). The HPP was almost the same under both conditions.
Vertical vocal cord movement showed no definitive differences with or without stimulation (
Table 2). However, the HIP of the vocal cord showed significantly more anterior displacement with stimulation (p<0.05).
Vertical and horizontal movements of the hyoid bone and the vocal cord with and without vertical stimulation on the suprahyoid and infrahyoid muscles (SIV).
Hyoid movement showed no significantly different results with and without stimulation during swallowing except for the HD of the hyoid (
Table 3). However, the VPP and HPP of the hyoid bone reached the same peak under both conditions. Vocal cord movement was the same for all kinematics with or without stimulation. Only the HIP of the vocal cord showed more anterior displacement with stimulation than without stimulation (p<0.05).
DISCUSSION
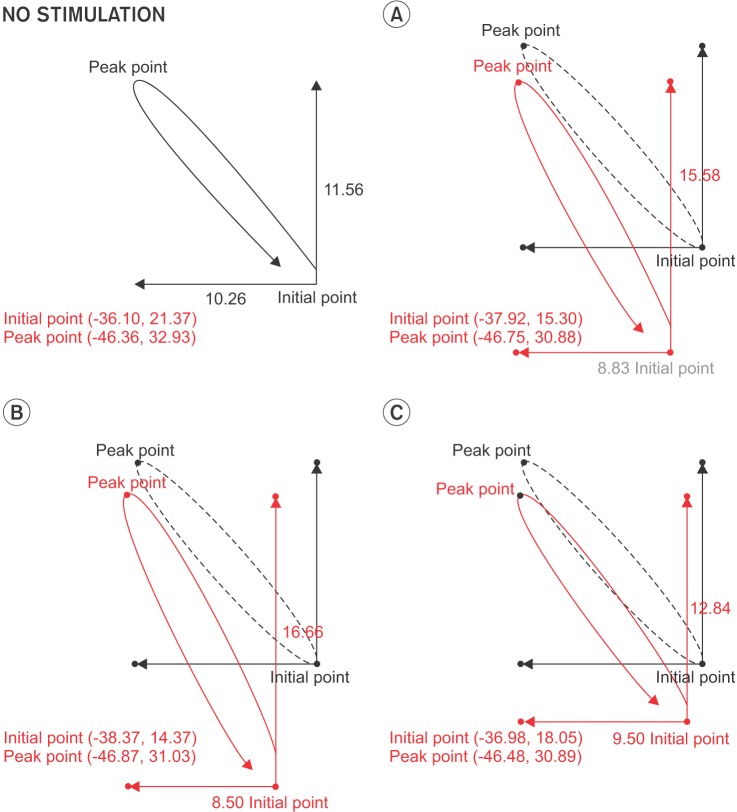
After development of the two-channel electrical stimulation system marketed under the name VitalStim, there was much debate about its benefits for patients with dysphagia. Evaluations of its physiological and clinical effects performed in several studies were inconclusive. Our study was designed to identify differences in the movement of the hyoid bone and the vocal cord with and without electrical stimulation in normal subjects, and to clarify physiological differences in swallowing in both conditions. In addition, we compared the effects of different arrangements of surface electrodes on the suprahyoid and infrahyoid muscles. In our study, stimulated swallows had the same peak position for the hyoid as nonstimulated swallows for all the placements tested (
Fig. 3). Therefore, we can conclude that stimulated swallowing is as safe as nonstimulated swallowing in healthy individuals.
Leelamanit el al. [
10] tested the hypothesis that the synchronous contraction of the thyrohyoid muscle by electrical stimulation during swallowing would improve dysphagia resulting from reduced laryngeal elevation. In this study, all participants had dysphagia due to reduced laryngeal elevation and were treated with electrical stimulation for up to 4 hours daily. The results demonstrated that stimulating synchronous contraction of the thyrohyoid muscle using a synchronous electrical stimulator during swallowing improved hyolaryngeal excursion. However, this study had several limitations in that it 1) did not explore the difficulty of specifically targeting the thyrohyoid without influencing neighboring muscles, 2) utilized an uncontrolled pretreatment duration, mixed etiology, age, and was an unblinded study and 3) failed to report specific data for physiologic findings.
Following the study by Ludlow et al. [
12], the theory that electrical stimulation may depress hyolaryngeal movement at rest for patients with dysphagia is now widely accepted. However, considerable disagreement remains on whether electrical stimulation depresses hyolaryngeal movement during stimulated swallowing in patients with dysphagia. Based on the conclusions drawn from this study, we disagree with this point. In our study, the main outcomes showed an initial downward displacement of the hyoid bone and different movements of the hyoid bone with the three placements used for electrical stimulation. The initial positions of the hyoid bone with SI and IO showed a downward and anterior displaced position; a result similar to that of Humbert et al. [
11]. During swallowing, the hyoid bone demonstrated more upward movement and less movement in the anterior direction and therefore, reached nearly the same peak position as the case without electrical stimulation.
Also, when we applied electrical stimulation using SIV, the hyoid bone movement did not show a significantly different position with electrical stimulation at rest compared with no electrical stimulation. Moreover, during swallowing, there were no significant differences in hyoid movement with and without stimulation. This finding demonstrated that electrical stimulation usually induces hyoid bone movement in the inferior direction during the initial stimulation and is in accord with the results from Humbert et al. [
11]. However, our results during swallowing were different from those of Humbert et al. [
11], with significant reductions in the hyoid bone peak elevation occurring during stimulated swallowing. Humbert el al. [
11] suggested that stimulated swallowing was less safe than non-stimulated swallowing. But in our study, stimulated swallows had the same peak position for the hyoid as did nonstimulated swallows in all the placements tested. Accordingly, it is clear that stimulated swallowing was as safe as nonstimulated swallowing in healthy individuals in this study. This finding demonstrated that the placement of the electrode with SI or IO was not important for hyoid bone movement and that both placements (SI and IO) could induce an initial depression and anterior movement of the hyoid bone.
Pearson et al. [
15] evaluated the structural properties of the suprahyoid muscles and concluded that the geniohyoid muscle contributes to the anterior movement of the hyoid bone, and that the mylohyoid muscle induced superior movement. In our study, anterior movement of the hyoid bone occurred regardless of electrode placement (SI, IO, SIV). Electrical stimulation of only the infrahyoid muscles without stimulating the suprahyoid muscles also induced anterior movement of the hyoid bone. Vocal cord movement was not significantly different between cases of electrical stimulation and no electrical stimulation. Only electrode placement with SI resulted in a more significant anterior movement during stimulated swallowing compared with nonstimulated swallowing.
Park et al. [
16] referred to the fact that effortful swallowing coupled with electrical stimulation increased the degree of hyoid elevation in healthy volunteers, but this effect faded within 2 weeks after treatment. They concluded that motor electrical stimulation of the infrahyoid muscles with effortful swallowing had a significant effect on the maximal vertical displacement of the larynx and used it as a means of resistance training. Also, Nam et al. [
17] investigated the effect of repeated sessions of electrical stimulation therapy on the neck muscles with respect to the stimulation site by using quantitative kinematic analysis of VFSSs in dysphagia patients with acquired brain injury. They concluded that different aspects of hyolaryngeal structural movements were increased according to the stimulation site utilized, and suggested that targeted electrical stimulation based on pathophysiology was necessary. However, neither study conducted a kinematic analysis of the activation of electrical stimulation. Rather, they evaluated the effects of repeated sessions of electrical stimulation therapy. Ultimately, our study did not demonstrate a difference in findings between different electrical placements (SI vs. IO vs. SIV, data not shown). Therefore, we conclude that a study of targeted electrical stimulation is required.
There are several limitations to this study. First, the sample size was too small. Second, this study was conducted in normal young healthy volunteers. Therefore, in older healthy volunteers or in patients with dysphagia, it is possible that the stimulation intensity, the power of effortful swallowing, and the skin status may be different. Berretin-Felix et al. [
18] suggested that a one-size-fits-all approach to electrical stimulation as an adjunctive modality in dysphagia rehabilitation may be misdirected. They also recommended additional research to modify the clinical application of electrical stimulation from patient to patient. Consequently, there is a need to study patients with dysphagia, of variable age, sex and so on. In addition, the Swallowing Kinematic Analysis that we used can be a useful tool for a patient to patient approach. Third, only 5 mL of fluid was used for VFSS. Logemann et al. [
19] reported that the maximal extent of structural anterior hyoid movement and laryngeal elevation was not significantly different with respect to the amount of fluid. Fourth, we evaluated the swallowing mechanism by the movement of the hyoid and larynx. Hyolaryngeal movement was determined as a surrogated value, but hyolaryngeal elevation aids laryngeal vestibule closure, which is important for airway protection. Thus, lack of normal hyolaryngeal elevation during swallowing can place individuals at risk for aspiration [
20]. Thus, it is worthy of evaluation. Furthermore, we evaluated functional values like penetration and aspiration based on the New VFSS Scale [
21]. The New VFSS Scale was clean in all participants and was therefore not listed separately in the results.
In conclusion, our study showed that electrical stimulation caused an initial depression of the hyoid bone, which reached nearly the same peak position during swallowing in healthy volunteers. The elevation of the hyoid bone was not dependent on the position of the electrode on the neck, such as on the infrahyoid or on both the supra and infrahyoid muscles. The only electrode position that did not have a significant effect on the movement of the hyoid bone was the vertical electrode.
Contrary to the results of previous studies, our motion analysis showed significant elevation of the hyoid bone during swallowing. Therefore, when used as resistance training, electrical stimulation during swallowing can be effective for producing a motor learning effect. In addition, further studies are necessary to identify the effect of electrical stimulation according to functional values in dysphagic patients.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download