Abstract
Objective
To analyze the differences in the vertical ground reaction force (GRF) variables of hemiplegic patients compared with a control group, and between the affected and unaffected limbs of hemiplegic patients using foot scans.
Methods
Patients (n=20) with hemiplegia and healthy volunteers (n=20) underwent vertical force analysis. We measured the following: the first and second peak forces (F1, F2) and the percent stances at which they occurred (T1, T2); the vertical force impulse (VFI) and stance times. The GRF results were compared between the hemiplegic patients and control individuals, and between the affected and unaffected limbs of hemiplegic patients. Additionally, we analyzed the impulse of the unaffected limb according to the motor assessment scale (MAS), Brunnstrom stage, and a Timed Up and Go Test.
Results
The F1s and F2s of the affected and unaffected limbs were significantly less than those of the normal control individuals (p<0.05). The T1s of both the affected and unaffected limbs of the patients were greater than control individuals, whilst the T2s were lower (p<0.05). Greater impulses and stance times were recorded on both sides of the patients than in the limbs of the control individuals (p<0.05). The MAS, Brunnstrom stage and Timed Up and Go Test results were significantly correlated with the VFI of the unaffected limbs (p<0.05).
A majority of stroke patients experience disabilities such as muscle weakness and tone changes, depending on the damaged area. Among the symptoms, the most common is gait disability, which causes independent activities of many patients to be restricted [1]. For these reasons, accurate diagnosis and treatment of gait disability are important goals of current rehabilitation therapy.
Various musculoskeletal complications develop during the gait treatment process of patients with hemiplegia. However, these complications develop on both the paralyzed and normal sides, and a number of other complications, such as ankle sprains, knee injuries, and/or foot fractures, can occur from excessive use of the musculoskeletal system [2]. When these complications occur, the rehabilitation period is prolonged and the chances of developing permanent disabilities increase. The occurrences of these complications demonstrate that the gait of a patient with hemiplegia applies abnormal force on both the paralyzed and normal sides. A study has shown that adverse effects in the musculoskeletal system can occur when this kind of excessive force is applied to the leg [3]. Current gait analysis methods performed on patients with hemiplegia use analysis data of the paralyzed leg as the basis for appropriate treatments during gait therapy; hence, they are an important part of the rehabilitation process. However, current gait therapies focus solely on the evaluation and treatment of the paralyzed leg, meaning that examinations of the normal leg are lacking.
To find accurate assessment and treatment methods for musculoskeletal problems that occur during rehabilitation therapy, methods for objectively assessing the force applied to the normal leg are needed. The F-Scan system can be used to assess the pressure applied to the leg during gait cycle and gait time, and can also be used to obtain the impulse of the force exerted on the legs during the stance phase. The impulse force applied to the legs can change depending on a patient's exercise capacities and degree of muscle recovery. By using the F-Scan assessments, gait problems can be identified according to a patient's status, thus helping clinicians choose the most appropriate treatment course.
In the present study, we conducted an F-Scan gait analysis of the paralyzed and normal legs of patients with hemiplegia. This was then compared with those of people with normal gaits to evaluate the pressure exerted and the sum of time and force on each foot. Whether the total force applied to the patient's normal leg was correlated with the level of recovery in mobility was also examined.
The study subjects consisted of stroke patients hospitalized in our department who had hemiplegia and did not show any balance abnormalities. Additional criteria included being ambulatory and capable of walking >8 m without resting. A total of 20 patients without prior leg injuries or surgeries were included in the patient group, and an additional 20 people without any gait abnormalities, prior history of neurological damage, or prior leg injuries or surgeries were included in the control group. Relevant factors such as patient age and weight were also assessed.
A foot scan system (F-Scan; Tekscan Inc., Boston, MA, USA) was used in the present study. The pressure and time applied to the feet during the stance phase of the gait cycle were measured. The assessment variables included: 1) the maximum pressure applied to the heels in the early stance phase (F1) and the elapsed time to this point (T1); 2) the maximum pressure applied during push-off in the late stance phase (F2) and the elapsed time to this point (T2); 3) total elapsed time in the stance phase; 4) impulse of force applied to the corresponding foot during the stance phase.
In the test, pressure insoles were fitted onto the patients' feet, and the patients were asked to walk around for 5 minutes in shoes with 1-cm heels for acclimatization prior to the actual test. The patients then stood on one leg for approximately 5 seconds, and the results were corrected by weight. The tests were performed on a hard, flat surface, and the measurements were made at each subject's normal gait speed (Fig. 1). Each subject had enough practice to adapt to walking while wearing the equipment; for accuracy, tests were performed five times, and the test result with a normal gait speed and stride was selected. For patients who used an orthosis, the test was performed after its removal.
Assessments including the Brunnstrom stage, the motor assessment scale (MAS), and Timed Up and Go Test were used to evaluate patient mobility. First, we used the Brunnstrom stage, which is used to assess the degree of mobility recovery; it is divided into a total of 6 stages consisting of the early stage without muscle tone, deep tendon reflex, or neurological responses; the stage exhibiting spasticity and a synergic pattern; and the stage of moving away from voluntary movements and synergic patterns [4].
Next, we used the MAS, which was performed to determine whether it could be used as a method for assessing the degree of recovery in walking and various motor skills in hemiplegic patients. This method consists of a total of nine categories including supine to side lying, supine to sitting over side of bed, balanced sitting, sitting to standing, walking, upper arm function, hand movements, advanced hand activities, and general tonus. A maximum of six points is given to each item, resulting in a total of 54 points used for motor skill degree measurement [5].
Finally, we used the Timed Up and Go Test to assess gait ability. This test begins with the subject sitting in a chair and evaluates the time required to stand up, walk around for a total of 3 m, return to the chair, and sit back down [6].
Statistical analysis was performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The one-way analysis of variance test was used to compare the differences in each of the measured parameters among the three groups (control, paralyzed side, and normal side). A post-hoc analysis by using the Tukey method was performed to identify groups demonstrating statistical differences. A correlation analysis was then performed in which the impulse value, MAS, and Brunnstrom stage of the normal leg of patients with brain damage were analyzed by using the Spearman coefficient, while the Timed Up and Go Test results were analyzed by using the Pearson coefficient. p-values of less than 0.05 were considered statistically significant.
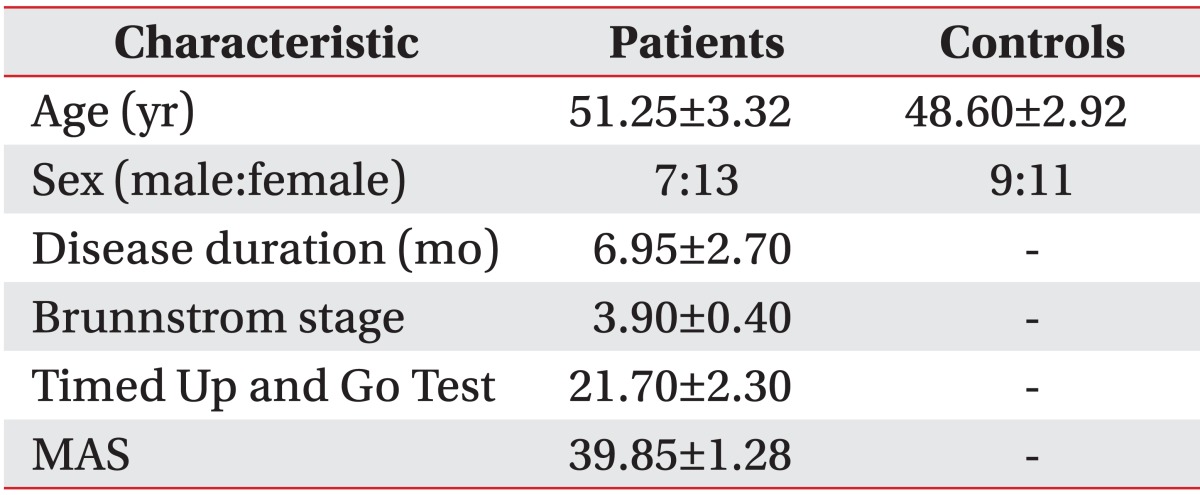
A total of 20 patients (seven men, 13 women; mean age, 51.25 years; mean time since stroke, 6.95 months) with hemiplegia and brain damage were included in the patient group, and 20 healthy adults (mean age, 48.6 years) were included in the control group. Age, sex, and time since stroke did not differ significantly between the groups (Table 1).
There were no significant differences in F1, F2, stance time, or impulse values between the left and right legs of the control group.
The F-Scan assessment results indicated that F1 and F2 measured in both paralyzed and normal legs of patients with brain damage differed significantly from those in the control group, whereas no significant differences were seen between the paralyzed and normal legs (p<0.05). The T1 value from the paralyzed and normal legs of the patient group were significantly increased compared to those of the control group (p<0.05). The T2 values of the paralyzed and normal legs of the patient group were significantly decreased compared to those of the control group (p<0.05) (Table 2).
Stance time was significantly shorter in the paralyzed and normal legs of the control group than in those of the patient group (p<0.05). The impulse value differed significantly among the control, paralyzed, and normal legs groups. In addition, significant correlations between the impulse values of the normal legs of the patient group and motor recovery were revealed by the Brunnstrom stage, MAS, and Timed Up and Go Test results (p<0.05) (Fig. 2).
Most stroke patients with hemiplegia develop a gait disability. However, the degree of gait disability varies due to differences in muscle weakness and muscle tone among patients [7]. A number of studies have objectively assessed the relationship of gait ability with various diseases [891011121314]. The most widely used methods involve the use of an F-Scan system or a force plate to measure the pressure and time duration applied to both legs [1516 17]. Several studies compared the pros and cons of the force plate and F-Scan methods, and Orlin and McPoil [18] indicated that use of a force plate has many disadvantages for patients with neurological abnormalities. Therefore, we used the F-Scan system in the present study. The usefulness of the ground reaction force (GRF) has been proven in a previous study. Mizrahi et al. [1920] measured various factors related to gait cycle time and distance and used them to prove the close relationships with their clinical assessment results. In addition, Wall and Ashburn [21] stated that the most basic method for gait analysis is the assessment of the categories related to gait time and distance. In the present study, various GRF categories were tested and compared to objectively assess gait disability in patients with hemiplegia. The time/pressure graph of normal GRF values obtained from the F-Scan shows a bimodal pattern. The part that shows the pressure increasing from the beginning is the phase in which the heel absorbs the shock as it touches the ground, while the latter half is the push-off phase in which thrust is generated (Fig. 3).
Studies have analyzed these GRF types and compared them to degrees of motor recovery. Chen et al. [22] classified GRF into four types, comparing gait speed to each variable by type. They proved that the group with greater mobility had faster gait speeds, and that the GRF graph model was similar to that of people with a normal gait [22]. However, this study's findings may reflect the researcher's subjective judgment. Therefore, instead of comparing these GRF types, objective values of various GRF test variables were used as standards in the present study.
F1 and F2 values of the paralyzed and normal legs displayed significant differences from those of people with a normal gait. This finding indicates that the shock absorption from using the heels in the early stance phase or push-off in the late stance phase does not occur normally. This can be attributed to an abnormal weight shift due to weakening of the leg muscles and the resulting instability, which is similar to the results shown in a previous study. Moreover, the T1 value was significantly longer in patients with brain damage than in people with a normal gait, whereas the T2 value was significantly shorter. Based on these results, patients with hemiplegia do not show perfect weight shift in both feet (paralyzed and normal), and they require more time in the stance phase than people with a normal gait; hence, they exhibit inefficient gait patterns.
Many previous studies focused on objectively assessing the pressure and time applied to the paralyzed leg to provide the appropriate treatment. Kerrigan et al. [23] examined the differences between paralyzed legs and normal legs in leg joint mobility during walking. However, during gait rehabilitation for stroke patients, complications including ankle sprains, ligament damage, and fractures occur on both the paralyzed and normal legs. Therefore, the paralyzed and normal legs must be assessed simultaneously.
In the present study, we compared the impulse of the pressure applied to paralyzed and normal legs. As shown in the study results, the impulse values of both the paralyzed and normal sides of patients were higher than those of normal people, and they were significantly higher on the normal side than on the paralyzed side. These results are consistent with the results of Horvath et al. [24] in which normal legs showed higher impulse values than paralyzed legs.
One reason for these results might be that a proper weight shift to the paralyzed leg did not occur, resulting in excessive weight loading in the normal leg. Furthermore, more time was required to move the paralyzed leg during the swing phase due to muscle weakness, increasing the stance time of the normal side and ultimately resulting in an increased impulse. However, Horvath et al. [24] examined patient groups and did not consider each patient's mobility. In patients with hemiplegia, the degree of gait disability corresponds to the degree of motor recovery, and musculoskeletal complications in these patients vary.
For these reasons, in the present study, we assessed various factors that can affect the mobility of patients with hemiplegia. First, the Brunnstrom stage was measured to assess the degree of motor recovery. Previous studies assessed the correlation between the Brunnstrom stage and gait. Chen et al. [22] also reported that the Brunnstrom stage, gait speed, and pattern were significantly correlated. The findings of the present study also demonstrated correlations between the Brunnstrom stage and impulses in the normal leg.
Tucak et al. [5] showed that the MAS from the initial hospitalization period significantly influenced the gait ability of stroke patients at discharge. In the present study, MAS was assessed during gait analysis, and its correlation with normal leg impulses was investigated. As a result, MAS and impulse showed significant correlations.
The Timed Up and Go Test, which is widely given to patients with stroke or leg fractures, was used here to assess basic gait ability. Studies on stroke patients indicated that more time was required to administer the test to stroke patients than to the control group [625]. In the present study, the Timed Up and Go Test results were significantly correlated with the impulse values of normal legs.
These results demonstrate that when the mobility of hemiplegic patients improves, the impulse values applied to the normal leg decrease. This is believed to be the result of improved mobility that causes an increase in the weight shift to the paralyzed leg, leading to a shorter swing phase in the paralyzed leg, which in turn reduces stance phase time and reduces the impulse applied to the normal leg. Based on these results, for hemiplegic patients with reduced mobility during gait training, the impulse applied to the normal leg can be reduced by actively engaging in weight shifting to the paralyzed leg, while the stance phase time in the normal leg can be shortened by strengthening the muscles in the paralyzed leg to shorten the swing phase time. Furthermore, reducing the impulse on the normal leg prevents various complications from occurring in the normal leg.
When the mobility of the paralyzed side of a patient with hemiplegia does not recover in the early gait training stage, it is common practice for clinicians to administer gait training with the use of an orthosis on the paralyzed side. However, our results indicate that the impulse applied on the normal leg is higher than that of paralyzed side, and thus the probability of complications such as factures and ankle joint sprains is also higher. The results from this study can be used to administer appropriate gait training programs that reduce the impulse of the force applied to both legs in order to prevent these complications.
Although differences based on motor recovery were observed when the impulse values from both legs were compared, additional studies were not conducted that evaluate the changes in these results and in these patients when appropriate rehabilitation therapy is administered. Therefore, in order to determine treatment efficacies, future studies are needed to compare the pre- and post-treatment results of patients with hemiplegia who use an orthosis or perform muscle-strengthening exercises.
References
1. Friedman PJ. Gait recovery after hemiplegic stroke. Int Disabil Stud. 1990; 12:119–122. PMID: 2096120.

2. Park JW, Park SH, Ko MH. Change of plantar fascia thickness in hemiplegic patients. J Korean Soc Phys Ther. 2009; 21:41–46.
3. Zadpoor AA, Nikooyan AA. The relationship between lower-extremity stress fractures and the ground reaction force: a systematic review. Clin Biomech (Bristol, Avon). 2011; 26:23–28.

4. Higashi T, Funase K, Kusano K, Tabira T, Harada N, Sakakibara A, et al. Motoneuron pool excitability of hemiplegic patients: assessing recovery stages by using H-reflex and M response. Arch Phys Med Rehabil. 2001; 82:1604–1610. PMID: 11689982.

5. Tucak C, Scott J, Kirkman A, Singer B. Relationships between initial Motor Assessment Scale scores and length of stay, mobility at discharge and discharge destination after stroke. NZ J Physiother. 2010; 38:7–13.
6. Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005; 86:1641–1647. PMID: 16084820.
7. Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003; 18:114–125. PMID: 12855307.

8. Bella GP, Rodrigues NB, Valenciano PJ, Silva LM, Souza RC. Correlation among the Visual Gait Assessment Scale, Edinburgh Visual Gait Scale and Observational Gait Scale in children with spastic diplegic cerebral palsy. Rev Bras Fisioter. 2012; 16:134–140. PMID: 22584771.
9. Brown CR, Hillman SJ, Richardson AM, Herman JL, Robb JE. Reliability and validity of the Visual Gait Assessment Scale for children with hemiplegic cerebral palsy when used by experienced and inexperienced observers. Gait Posture. 2008; 27:648–652. PMID: 17913500.

10. Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007; 88:1410–1415. PMID: 17964880.

11. Sutherland DH. The evolution of clinical gait analysis part l: kinesiological EMG. Gait Posture. 2001; 14:61–70. PMID: 11378426.

12. Takebe K, Basmajian JV. Gait analysis in stroke patients to assess treatments of foot-drop. Arch Phys Med Rehabil. 1976; 57:305–310. PMID: 1084734.
13. Tenore N, Fortugno F, Viola F, Galli M, Giaquinto S. Gait analysis as a reliable tool for rehabilitation of chronic hemiplegic patients. Clin Exp Hypertens. 2006; 28:349–355. PMID: 16833045.

14. Turani N, Kemiksizoglu A, Karatas M, Ozker R. Assessment of hemiplegic gait using the Wisconsin Gait Scale. Scand J Caring Sci. 2004; 18:103–108. PMID: 15005669.

15. Bertani A, Cappello A, Benedetti MG, Simoncini L, Catani F. Flat foot functional evaluation using pattern recognition of ground reaction data. Clin Biomech (Bristol, Avon). 1999; 14:484–493.

16. Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005; 22:51–56. PMID: 15996592.

17. Morita S, Yamamoto H, Furuya K. Gait analysis of hemiplegic patients by measurement of ground reaction force. Scand J Rehabil Med. 1995; 27:37–42. PMID: 7792548.
19. Mizrahi J, Susak Z, Heller L, Najenson T. Objective expression of gait improvement of hemiplegics during rehabilitation by time-distance parameters of the stride. Med Biol Eng Comput. 1982; 20:628–634. PMID: 7176722.

20. Mizrahi J, Susak Z, Heller L, Najenson T. Variation of time-distance parameters of the stride as related to clinical gait improvement in hemiplegics. Scand J Rehabil Med. 1982; 14:133–140. PMID: 7134913.
21. Wall JC, Ashburn A. Assessment of gait disability in hemiplegics. Hemiplegic gait. Scand J Rehabil Med. 1979; 11:95–103. PMID: 493898.
22. Chen CY, Hong PW, Chen CL, Chou SW, Wu CY, Cheng PT, et al. Ground reaction force patterns in stroke patients with various degrees of motor recovery determined by plantar dynamic analysis. Chang Gung Med J. 2007; 30:62–72. PMID: 17477031.
23. Kerrigan DC, Karvosky ME, Riley PO. Spastic paretic stiff-legged gait: joint kinetics. Am J Phys Med Rehabil. 2001; 80:244–249. PMID: 11277129.
24. Horvath M, Tihanyi T, Tihanyi J. Kinematic and kinetic analyses of gait patterns in hemiplegic patients. Facta Univ Ser Physic Educ Sport. 2001; 1:25–35.
25. Faria CD, Teixeira-Salmela LF, Silva EB, Nadeau S. Expanded timed up and go test with subjects with stroke: reliability and comparisons with matched healthy controls. Arch Phys Med Rehabil. 2012; 93:1034–1038. PMID: 22381595.

Fig. 1
A hemiplegic patient who is performing gait analysis with insoles in shoes and with a preamplifier attached to ankle with Velcro (left) and main component of the F-Scan system (right).
Fig. 2
(A) Timed Up and Go Test showed a positive correlation, (B) motor assessment scale, and (C) Brunnstrom stage showed a negative correlation with vertical force impulse. IMP, impulse; N, newton; BW, body weight (kg).
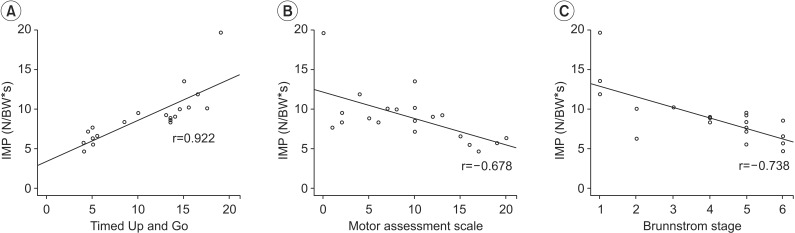
Fig. 3
Graph demonstrating the selected components of the vertical ground reaction force-time curve during stance phase. N, newton; BW, body weight (kg); F1, peak force at the moment of foot flat; F2, peak force at the moment of toe off; T1, time between the moment of heel contact and foot flat; T2, time until the toe off; Impulse, area under the curve.
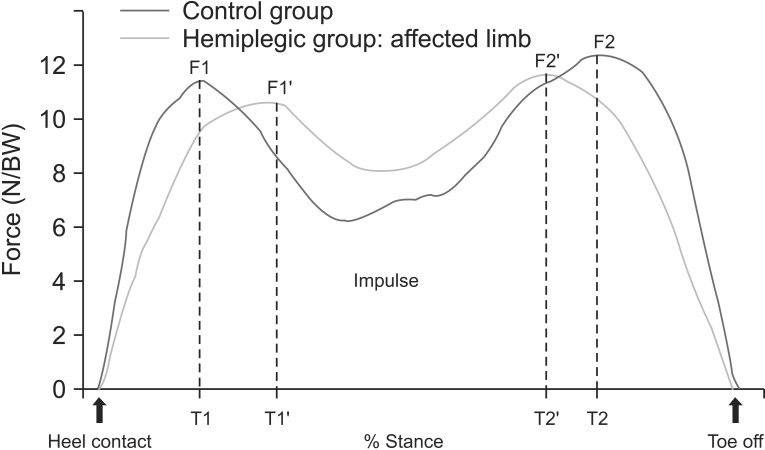
Table 2
Vertical ground reaction measures
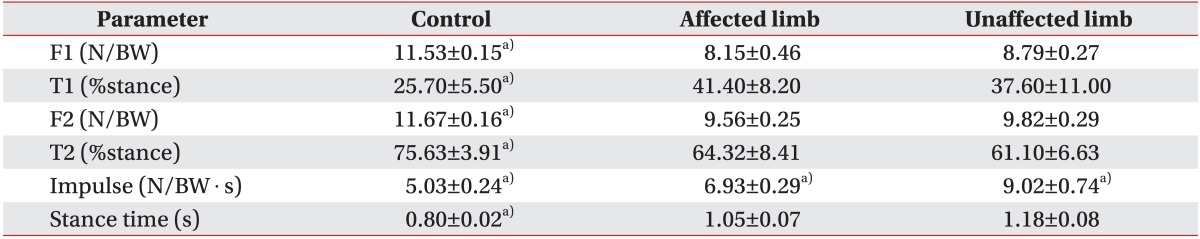
Values are presented as mean±standard deviation.
F1, peak force at the moment of foot flat; N, newton; BW, body weight (kg); F2, peak force at the moment of toe off; T1, time between the moment of heel contact and foot flat; T2, time until the toe off.
a)p<0.05 from one-way analysis of variance test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download