INTRODUCTION
Head and neck cancer (HNC) can arise in anatomical regions related to swallowing, but the incidence of aspiration varies [
1]. Aspiration can occur not only due to the tumor itself, but also due to surgery, radiotherapy, and chemotherapy [
2]. Because dysphagia affects morbidity and mortality in HNC, it is important that its risk factors be identified so that it can be carefully monitored in clinical practice. Some studies have attempted to identify the risk for aspiration in patients with HNC [
3,
4]. The few studies that have been conducted on dysphagia in HNC patients have focused on HNC subtypes other than tongue cancer. HNC contains several cancer subtypes with different anatomical lesions. Because their characteristics are heterogeneous, it is not easy to predict risk factors for dysphagia in the entire population of HNC patients.
Tongue cancer is the most frequent intraoral HNC. In oral cancer, dysphagia is caused by extensive tissue loss, limited excursion of the remaining tissue, and sensory paralysis of the tongue, soft palate, and pharynx [
5]. The swallowing function in HNC patients may be affected by the degree of resection and the nature of reconstruction. Although the oral stage of the swallow is generally more severely impaired, the pharyngeal stage of the swallow may be affected if resection includes the tongue base [
6,
7]. Previous studies on dysphagia in oral cancer patients were conducted on a small sample size or lacked radiologic evaluation. A videofluoroscopic swallowing study (VFSS) to evaluate aspiration is needed in patients with HNC who have these risk factors [
3].
A VFSS of the swallowing process was conducted to identify risk factors for dysphagia in patients with tongue cancer. Dysphagia characteristics were also compared before and after surgery.
Go to :

RESULTS
VFSS findings
VFSS was performed on 74 patients before surgery and on 87 patients after surgery (
Table 2). In the oral phase of swallowing, there were no patients with inadequate lip movement before surgery. Patients who underwent VFSS after surgery were observed to have lip movement abnormalities, but this was not statistically significant. In the oral phase, the differences in tongue control, chewing, and oral transit time in patients who were analyzed by VFSS before and after surgery were statistically significant.
Table 2
Comparison of videofluoroscopic swallowing study findings before and after surgery in tongue cancer patients
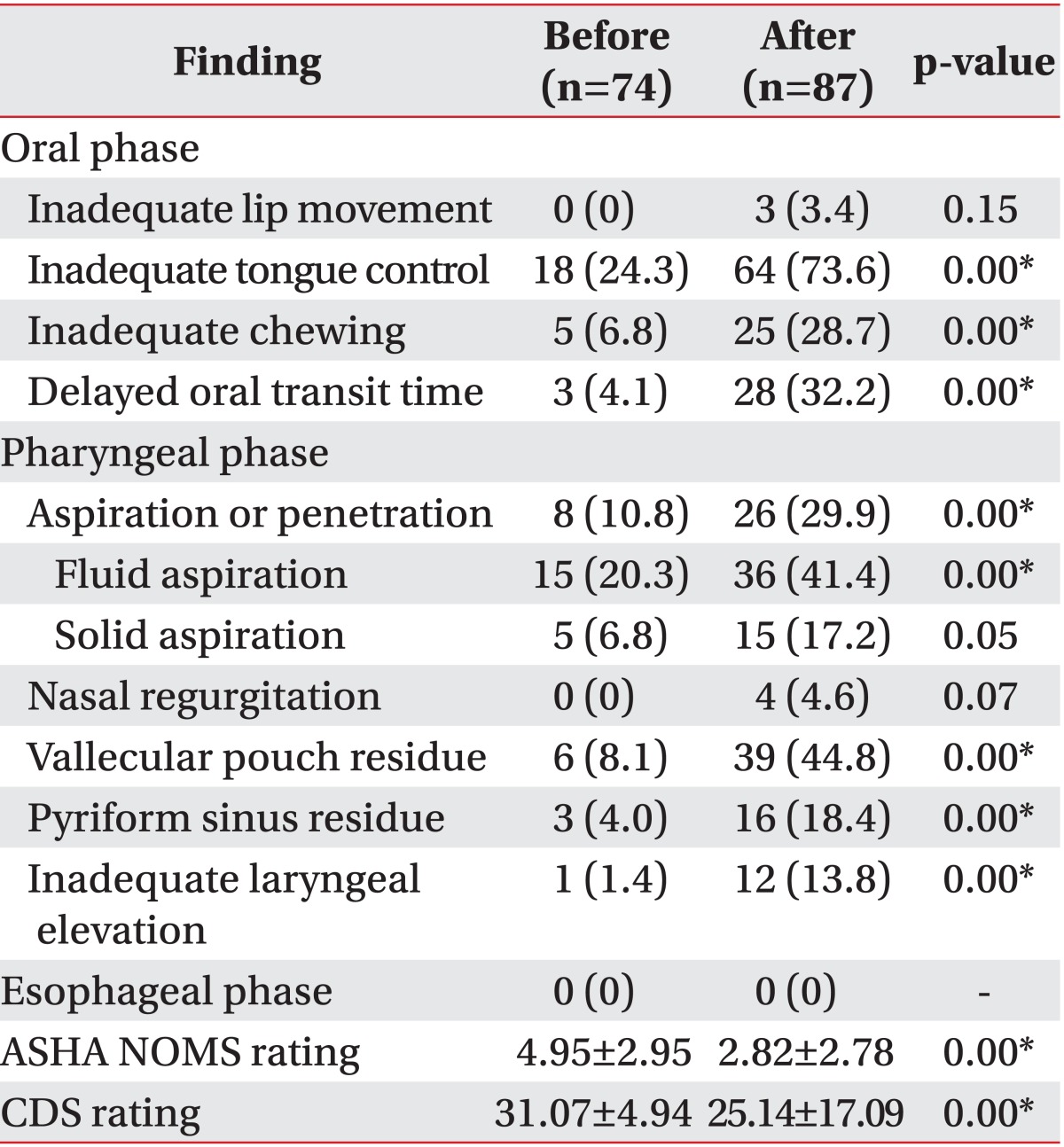

In the pharyngeal phase, aspiration or penetration was observed in eight patients (10.8%) who underwent VFSS before surgery, and in 26 patients (29.9%) who underwent VFSS after surgery. Among patients with aspiration, fluid aspiration was observed significantly higher in patients before surgery than after surgery. Solid aspiration showed significant differences between the two groups. While four patients (4.6%) showed nasal regurgitation after surgery, nasal regurgitation was not observed in any patient before surgery. Vallecular pouch residue was observed in six patients (8.1%) before surgery and in 39 patients (44.8%) afterward. Pyriform sinus residue was observed in three patients (4.0%) before surgery and in 16 (18.4%) after surgery. Inadequate laryngeal elevation was observed in only one (1.4%) patient who received VFSS before surgery, but was seen in 12 (13.8%) patients who underwent VFSS after surgery. Among the pharyngeal phase abnormalities, aspiration and penetration, fluid aspiration, vallecular pouch residue, pyriform sinus residue, inadequate laryngeal elevation, and epiglottic closure were statistically different between the two groups.
No patients had esophageal phase abnormalities, either before or after surgery. The rating on the American Speech-Language-Hearing Association (ASHA) National Outcome Measurement System (NOMS) swallowing level scale was 4.95±2.95 in the patients who underwent VFSS before surgery and 2.82±2.78 in those who underwent VFSS afterward. The clinical dysphagia scale rating was 31.07±4.94 before surgery and 25.14±17.09 after surgery. Both the ASHA NOMS and clinical dysphagia scale (CDS) ratings were significantly different between the two groups. These findings were not different according to the period of time between the surgeries and when the VFSS was conducted.
VFSS was performed on 28 patients both before and after surgery.
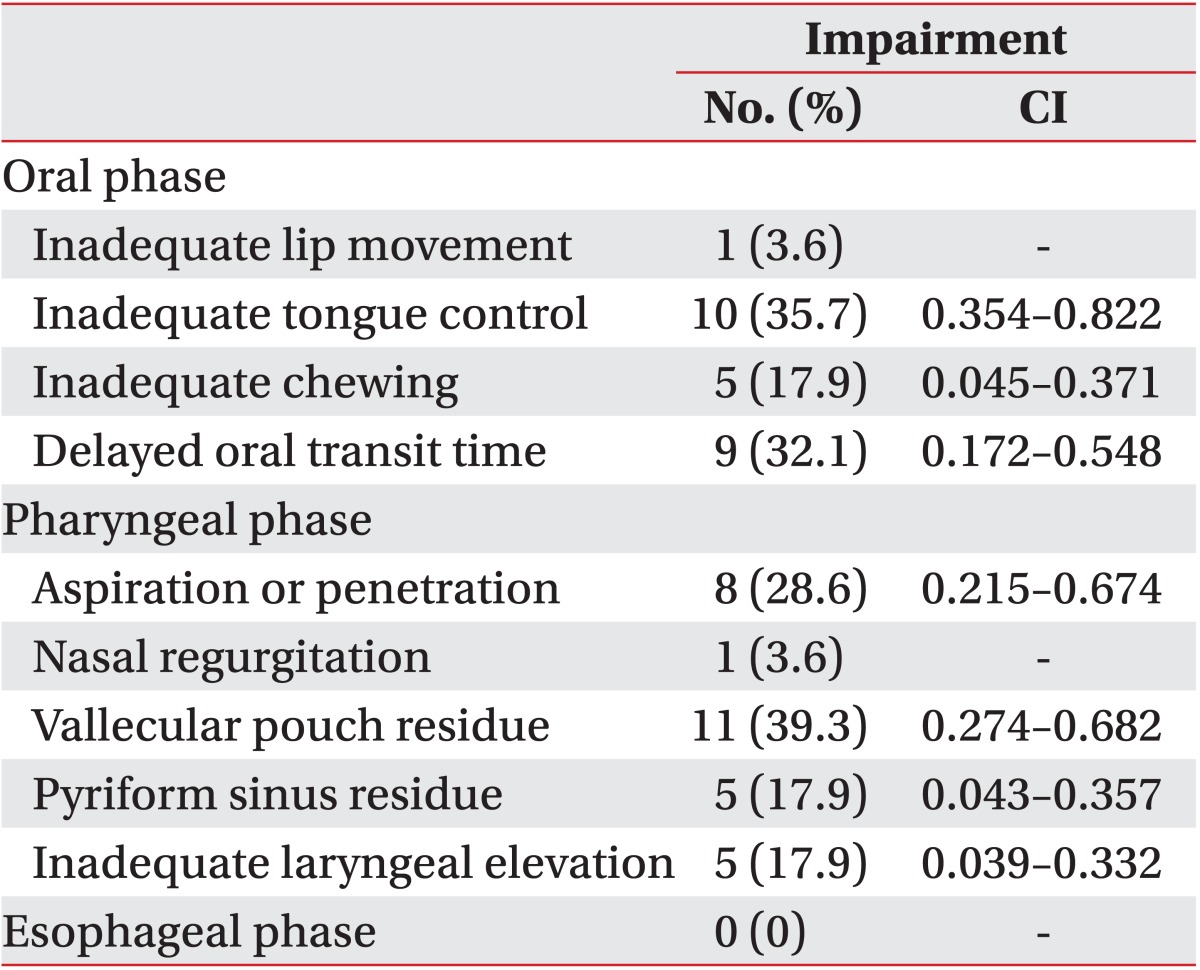
Table 3 shows the proportion of patients exhibiting each impairment after surgery, though none of the patients exhibited any of these impairments prior to surgery. Abnormal tongue control was observed in 10 patients (35.7%). Chewing abnormalities developed in five patients (17.9%), and delayed oral transit time was observed in nine patients (32.1%). In the pharyngeal phase, eight patients (28.6%) showed newly developed aspiration or penetration. Vallecular pouch residue was observed in 11 patients (39.3%), and pyriform sinus residue and aggravation of laryngeal elevation emerged in five patients (17.9%).
Table 3
Aggravation of videofluoroscopic swallowing study findings before and after surgery

Risk factors associated with aspiration in tongue cancer patients after surgery
Table 4 shows univariate and multivariate analyses of risk factors for aspiration after surgery. Aspiration occurred at a significantly higher rate in males, patients with left tongue tumors, patients who underwent more extensive tumor resection surgery (hemi- or total glossectomy), patients with lymph node metastasis (N1 or N2), and patients who required more extensive lymph node dissection (MRND). However, age, extent of reconstruction, tumor stage, radiotherapy, and chemotherapy had no significant impact on the aspiration risk in patients after surgery.
Table 4
Univariate and multivariate analyses for risk factors related to aspiration after surgery
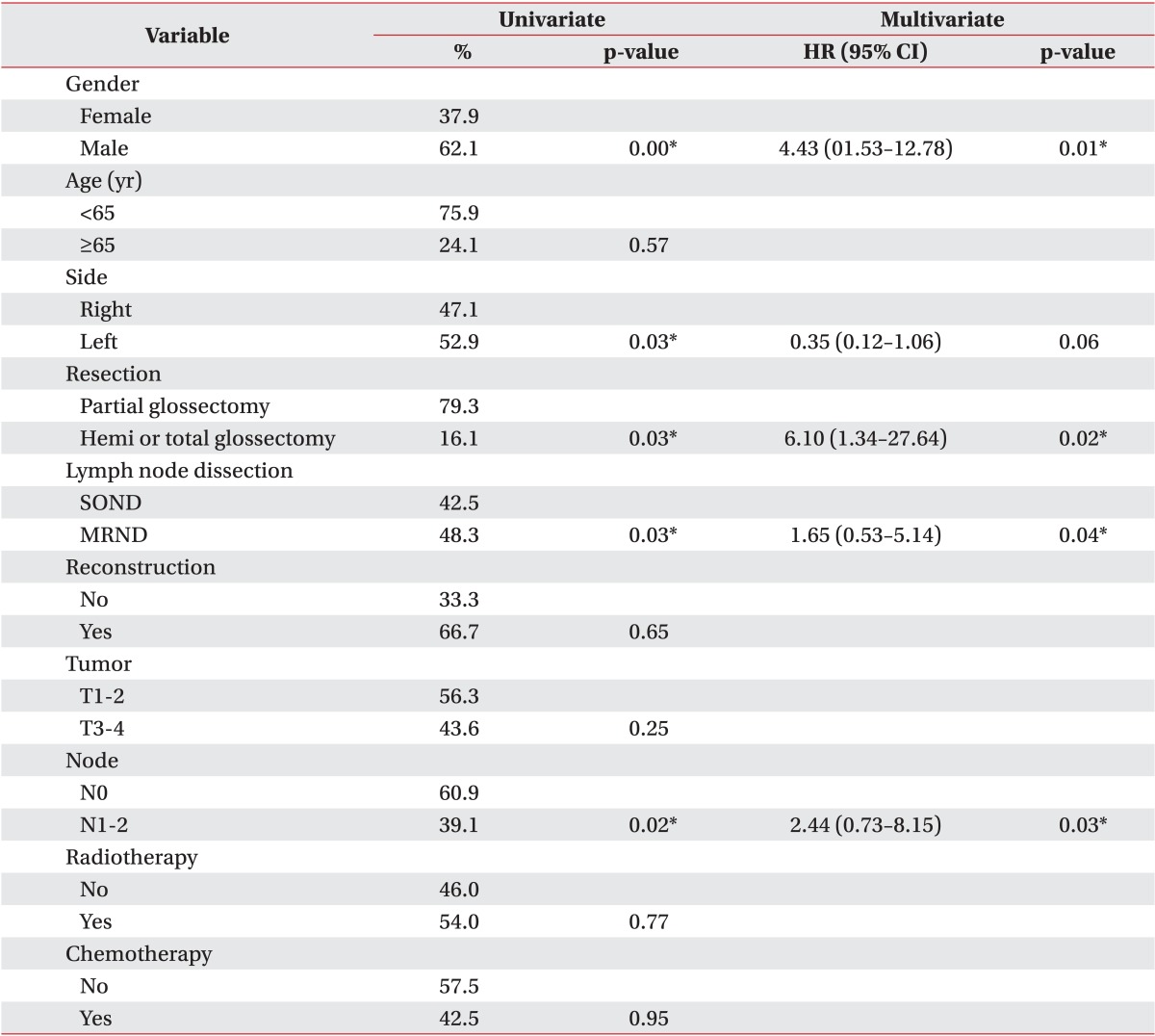

Multivariate logistic regression analyses revealed that male gender, hemi- or total glossectomy, lymph node metastasis (N1 or N2), and more extensive lymph node dissection (MRND) put patients after surgery at a higher risk of developing aspiration. The incidence of aspiration was 4.43 times higher in male patients than in female patients (hazard ratio [HR], 4.435; 95% confidence interval [CI], 1.53-12.78). The incidence of aspiration was 6.10 times higher in patients who required hemi- or total glossectomy than in those who only required partial glossectomy (HR, 6.103; 95% CI, 1.34-27.64). The incidence of aspiration was 1.65 times higher in patients who underwent MRND than in those who underwent SOND (HR, 1.652; 95% CI, 0.53-5.14). The incidence of aspiration was 2.44 times higher in N1 and N2 patients than in patients with no metastasis (HR, 2.443; 95% CI, 0.73-8.15).
When we conducted analyses of risk factors with aspiration in tongue cancer patients before surgery, there were no statistically significant risk factors. The results were the same when we conducted the analyses of risk factors in the full 133 patients.
Go to :

DISCUSSION
This study intended to identify risk factors for dysphagia in patients with tongue cancer. To do this, we conducted a VFSS for the swallowing process and compared dysphagia characteristics before and after surgery.
Tongue cancer patients experience difficulties in the pharyngeal phase of swallowing as well as in the oral phase. Patients who underwent VFSS after surgery had a higher incidence of inadequate tongue control and chewing, and delayed oral transit time in the oral phase. In the pharyngeal phase, aspiration and penetration, especially during fluid swallowing, were observed more frequently in the patients who underwent VFSS after surgery. Moreover, there were significant increases in inadequate laryngeal elevation, vallecular pouch residue, and pyriform sinus residue after surgery. In patients who VFSS was conducted both before and after surgery, significant differences in tongue control, chewing, oral transit time, laryngeal elevation, vallecular pouch residue, pyriform sinus residue, and aspiration and penetration were observed after surgery.
There were limitations because this data included patients who underwent VFSS only once. So, we compared VFSS findings from the patients performed both before and after surgery separately, too. The results showed that tongue control and oral transit time were more aggravated than other parameters in the oral phase. In the pharyngeal phase, aspiration and residue of the vallecular pouch were aggravated in particular.
Post-swallow residue is widely considered to be a sign of swallowing impairment and is assumed to pose a risk for aspiration on subsequent swallows. Post-swallow residue in one or both pharyngeal spaces was significantly associated with impaired swallowing safety on the subsequent clearing swallow for the same bolus [
11].
Aspiration can be caused by numerous factors, including surgery, radiotherapy, chemotherapy, and abnormal motility of swallowing-related structures. Tumor resection can damage the structures that control swallowing, radiotherapy may cause scar tissue formation, and chemotherapy can result in abnormal motility of the muscles involved in swallowing due to cell apoptosis [
12,
13]. Aspiration was observed in 29.9% of tongue cancer patients in this study, which is lower than that seen in previous studies of HNC (36%-94%) [
1,
4,
14,
15,
16,
17]. This may be because HNC includes variable cancers related with anatomical structures involved during swallowing.
When food enters the oral cavity, the oral preparatory phase starts with manipulation of the bolus. Mechanoreceptor cells, which are concentrated on the tip of the tongue and the center of the palate, provide information concerning the position and size of the food bolus via the trigeminal nerve. The oral transport phase begins with peristaltic movements of the tongue that stimulate mechanoreceptors in the hard palate. The involuntary pharyngeal phase starts with the contraction of the genioglossus or mylohyoid muscles and involves the cooperative action of the suprahyoid muscles [
18]. Aspiration was observed at a significantly higher rate in patients who underwent hemi- or total glossectomy than in those who underwent partial glossectomy. Previous studies have reported an incidence of aspiration in 10%-37% of patients who undergo a total glossectomy [
19]; however, those studies did not evaluate differences according to the extent of surgery. Lango et al. [
20] reported that tissue fibrosis and edema were aggravated after surgery. More extensive surgery leads to injury of structures related with swallowing, which leads to the occurrence of tissue fibrosis and edema.
Gender was significantly related to aspiration incidence in tongue cancer patients specifically. By contrast, in HNC overall, gender was not related to aspiration [
3]. The correlation between gender and aspiration has not been broadly examined in the dysphagia field. Any biological explanation for this gender difference is absent. It is same, not only in the cancer population, but also in the general population [
21]. In this study, male gender was an increased risk factor for aspiration in tongue cancer patients. Perhaps women are more assertive in requesting treatment and are more likely to receive a recommendation from their physician. Further research is needed to clarify the finding.
Chemotherapy is a principal predictive factor of aspiration in HNC. A systemic review demonstrated that reduced laryngeal excursion, base-of-tongue dysfunction, reduced pharyngeal contraction, and impaired epiglottic movement were most frequently reported [
22]. However, in this study, there were no statistically significant differences in the incidence of aspiration between patients who received chemotherapy and those who did not. Radiotherapy was also not a predictive factor in tongue cancer patients. It will be necessary to compare radiotherapy and chemotherapy separately in a sufficient number of patients who received either chemotherapy or radiotherapy, because many patients received both in this study.
Patients with an N1 or N2 stage had a higher incidence of aspiration than patients with an N0 stage. Furthermore, patients who underwent MRND had a higher incidence of aspiration than those who underwent SOND. Lymph node metastasis implies a more advanced disease, which requires more extensive resection. It can therefore be assumed that patients with a high nodal stage were more likely to have undergone MRND in this study.
The main limitation of this study was that only 28 patients underwent VFSS both before and after surgery. In addition, treatment modalities varied depending on tumor stage. Moreover, this study did not enroll all patients with tongue cancer, only those who were referred to our clinic for VFSS, which may introduce a selection bias. Further studies with a larger sample size should be performed in tongue cancer patients both before and after surgery.
In conclusion, tongue cancer patients experienced difficulties in the pharyngeal phase as well as the oral phase of swallowing. The difficulties were found to worsen after tongue cancer surgery. Male gender, extensive tumor resection, higher node stage, and more extensive lymph node dissection were major risk factors for aspiration in tongue cancer patients. Physicians should be aware of the characteristics of dysphagia in tongue cancer patients in order to effectively direct subsequent swallowing therapy.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download